A rapid quantitative turbimetric d-dimer assay has high sensitivity for detection of pulmonary embolism in the ED
Affiliations
- Department of Emergency Medicine, Advocate Christ Medical Center, Oak Lawn, Illinois, USA
Correspondence
- Address reprint requests to Erik B. Kulstad, MD, MS, 4440 W. 95th St., Oak Lawn, IL 60453 USA

Affiliations
- Department of Emergency Medicine, Advocate Christ Medical Center, Oak Lawn, Illinois, USA
Correspondence
- Address reprint requests to Erik B. Kulstad, MD, MS, 4440 W. 95th St., Oak Lawn, IL 60453 USA
Affiliations
- Department of Emergency Medicine, Advocate Christ Medical Center, Oak Lawn, Illinois, USA
Affiliations
- Department of Emergency Medicine, Advocate Christ Medical Center, Oak Lawn, Illinois, USA
To view the full text, please login as a subscribed user or purchase a subscription. Click here to view the full text on ScienceDirect.
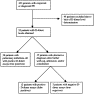
FIGURE 1
Flow diagram of study subjects.
Abstract
Many rapid d-dimer assays are commercially available with wide ranges of reported sensitivities, often based on small sample sizes. This has limited their intended use as rapid and inexpensive tests to evaluate pulmonary embolism in the low-risk patient. We sought to determine the sensitivity of the STA-Liatest D-Di d-dimer assay in our ED. We performed a retrospective analysis of 103 patients seen in our ED with the admitting diagnosis of known or suspected pulmonary embolism. These charts were assessed to establish if a d- dimer assay was performed within 24 hours. These charts were then reviewed to determine what diagnostic studies were performed and what final diagnosis was reached. Of the 103 charts identified, 55 had d-dimer assays performed within 24 hours. Of those, 38 were diagnosed with pulmonary embolism; none had negative d-dimer assays (<400 ng/mL). Using the exact method, the sensitivity of this assay was calculated to be 100% with a 95% confidence interval (CI) of 91.4% to 100%. Our results suggest that the STA-Liatest D-Di d-dimer assay could have an adequate sensitivity to be used to rule out pulmonary embolism in low-risk patients. Further prospective studies with larger sample sizes are required to validate this observation.
To access this article, please choose from the options below
Purchase access to this article
Claim Access
If you are a current subscriber with Society Membership or an Account Number, claim your access now.
Subscribe to this title
Purchase a subscription to gain access to this and all other articles in this journal.
Institutional Access
Visit ScienceDirect to see if you have access via your institution.
Related Articles
Searching for related articles..


