Does intraosseous equal intravenous? A pharmacokinetic study
Original Contribution
Does intraosseous equal intravenous? A pharmacokinetic studyB,BB

Daniel D. Von Hoff MD, FACPa, John G. Kuhn Pharm D, FCCP, BCOPb,
Howard A. Burris III MD, FACPc, Larry J. Miller MDd,*
aTranslational Genomics Research Institute and Arizona Cancer Center, University of Arizona, Phoenix, AZ 85721, USA
bUniversity of Texas Health Science Center at San Antonio, San Antonio, TX 78249, USA
cCentennial Medical Center, Nashville, TN 37203, USA
dVidacare Corporation, San Antonio, TX 78216, USA
Received 28 September 2006; revised 20 March 2007; accepted 21 March 2007
Abstract
Study Objective: Despite the growing popularity of Intraosseous infusion for adults in emergency medicine, to date there has been little research on the pharmacokinetics of intraosseously administered medications in humans. The objective of the study was to compare the pharmacokinetics of intraosseous vs intravenous administration of morphine sulfate in adults.
Methods: The study followed a prospective, randomized, Crossover design. Each subject was equipped with an indwelling intraosseous access device and an intravenous line. Subjects were randomized to receive a 5-mg bolus of morphine sulfate infused intraosseously or intravenously, followed by the alternate administration route 24 hours later.
Serial venous blood samples (5 mL) were taken at baseline and at 13 time points over 8 hours postinfusion. Blood samples were analyzed for morphine concentration by radioimmunoassay. Pharmacokinetic parameters were calculated from the data, including maximum plasma concentration (Cmax), Time to maximum concentration (Tmax), and area under plasma concentration-time curve (AUC), among others. Data were analyzed by analysis of variance.
Results: No statistically significant differences were observed between intraosseous and intravenous administration of morphine sulfate for nearly all of the pharmacokinetic parameters including Cmax (235 F 107 vs 289 F 197 ng/mL, mean F SD, IO vs IV, respectively), Tmax (1.3 F 0.5 vs 1.4 F 0.5 minutes), and AUC(0-l) (4372 F 1785 vs 4410 F 1930 ng min-1 mL-1). There was, however, a
Presentation Information: The study was presented at the National Association of Emergency Medical Services Physicians annual meeting in Naples, FL, January 2005.
B Funding/Financial Interest: The study was funded by a grant from LifeQuest Medical. At the time of the study, Larry Miller was the CEO of LifeQuest
Medical. Doctor Kuhn and Dr Von Hoff were shareholders of LifeQuest Medical. Doctor Burris was principal investigator.
BB Author Contribution Statement: D. V. H. and J. K. conceived the study, designed the trial, and supervised conduct of the trial. D. V. H., J. K., and
L. M. supervised the data collection. H. B. III, D. V. H., and J. K. were responsible for recruitment of participating centers and patients. The study nurse at LifeQuest Medical managed the data, including quality control. D. V. H. and J. K. drafted the manuscript, and L. M. contributed substantially to the revision, adding the implications for emergency medicine and prehospital emergency care. J. K. takes responsibility for the article as a whole.
* Corresponding author. Tel.: +1 210 375 8500; fax: +1 210 375 8537.
E-mail address: [email protected] (L.J. Miller).
0735-6757/$ - see front matter D 2008 doi:10.1016/j.ajem.2007.03.024
statistically significant difference in the volume of distribution in the central compartment, Vd ( P =
.0247), which in the opinion of the investigators was thought to be due to a minor deposition effect near the intraosseous port or in the bone marrow.
Conclusion: The results support the bioequivalence of intraosseous and intravenous administration of morphine sulfate in adults.
D 2008
Introduction
Background
The intraosseous space of the bones has been used to administer fluids and medications in emergency medical situations since World War II [1]. In pediatric medicine, intraosseous infusion has long been the standard of care when traditional intravenous access is difficult or impos- sible [2].
Advances in medical device technology over the past few years have resulted in a number of devices that greatly facilitate intraosseous infusion of drugs and fluids in adults. Such devices penetrate through the hard cortex of adult bone to the highly vascularized intraosseous space by means of specially designed needles or needle sets. As a result of the introduction of these devices, the 2005 Advanced Cardiac Life Support guidelines of the American Heart Association now specifically recommend intraosseous infusion for adults when intravenous access is unavailable [3]. The guidelines state that intraosseous administration is safe, effective, and equivalent to intravenous administration.
As the life-saving capability of intraosseous infusion becomes well known among emergency medical personnel, a frequently asked question is whether intraosseous infusion is equivalent to conventional intravenous infusion. Although the physiologic and pharmacodynamic effects of intra- osseously administered fluids and medications have been widely observed and documented for decades [4-9], few studies compare the pharmacokinetics of medications ad- ministered via the intraosseous route to intravenous infusion. The studies that do exist were conducted in anesthetized animal models [10-16]. To date, no study has compared the pharmacokinetics of intraosseously administered medication to intravenously administered medication in conscious human subjects. The growing popularity of intraosseous infusion for adults necessitates a validation of the pharma- cokinetic profile of intraosseously administered medications relative to traditional intravenous administration.
Goal of this investigation
Accordingly, the goal of this investigation was to compare the pharmacokinetics of intraosseously vs intrave- nously administered morphine sulfate in human subjects. For several reasons, it was not feasible to conduct such a study in healthy volunteers or emergency patients using available adult intraosseous devices. As such, the inves-
tigators used implanted intraosseous devices for the administration of drugs in patients with cancer who had previously failed at least one attempt at conventional intravenous access. Morphine sulfate was chosen as the study drug because some of the study patients required analgesics. Pharmacokinetic parameters calculated from serum levels of morphine in this study therefore served as Surrogate markers for expected drug levels in emergency patients. Primary outcome measures were standard pharma- cokinetic parameters, including maximum plasma concen- tration (Cmax), time to maximum plasma concentration (Tmax), and area under the concentration-time curve (AUC).
Methods
Study design
The study design was a Latin square crossover, with each subject serving as his or her own control. Subjects received a single dose of morphine sulfate administered through an implanted intraosseous port (described below) or through a standard intravenous line, followed by a second dose of morphine sulfate via the alternate administration route no sooner than 24 hours later. Serial blood samples were collected via a second intravenous line. Samples were collected before dosing (baseline); at the end of infusion (1 minute); at 2, 5, 10, 15, 30, 45, 60, 90 minutes; and at 2, 3,
4, 6, and 8 hours postinfusion. Plasma samples were subsequently analyzed for morphine sulfate concentration. Standard pharmacokinetic parameters for both intraosseous and intravenous administration were then calculated from the concentration values.
Setting
The study was conducted on inpatients at 8 clinical centers across the country: St. Luke’s Lutheran Hospital (San Antonio, TX), Arlington Cancer Center (Arlington, TX), Huntsville Hospital (Huntsville, AL), the University of Arizona Medical Center (Tucson, AZ), Brooke Army Medical Center at Fort Sam Houston (San Antonio, TX), the University of Texas Health Science Center (San Antonio, TX), the University of Southern California Medical Center (Los Angeles, CA), M.D. Anderson Cancer Center (Houston, TX), and Lutheran General Hospital (Park Ridge, IL). The study protocol was approved by the Institutional Review Boards at all 8 study centers.
Selection of participants
Individual study sites were responsible for recruiting and enrolling patients. The study was conducted in patients with cancer because these patients often suffer from lack of venous access and need supportive medications, including morphine sulfate, which can only be administered intrave- nously. To be eligible for inclusion in the study, subjects had to be at 18 years of age or older, with a confirmed diagnosis of cancer. Subjects had to have previously failed at least one attempt with a conventional intravenous access device (defined as vein inaccessibility, clotting of the vein or intravenous line, infection, or extravasation necrosis around the intravenous line). Subjects had to have adequate Organ function (bone marrow, liver, kidney) confirmed by laboratory tests and a normal chest x-ray within 2 weeks before device implantation. All subjects provided written informed consent before participating in the study.
The intraosseous infusion device was implanted in the Iliac crest of the pelvis. As such, subjects were ineligible for inclusion in the study if they exhibited any condition that rendered the iliac crest unsuitable for implantation, such as multiple myeloma, advanced osteoporosis, osteogenesis imperfecta, or tumor involvement in this area of the pelvis (determined by computed tomography or magnetic reso- nance imaging scan within 21 days before implantation.) In addition, subjects had to have an iliac crest at least 10.0 mm wide at the implantation site and a maximum distance from the skin to the implantation site of no more than 2.5 cm (determined by computed tomography or magnetic reso- nance imaging scan).
Subjects were also ineligible for the study if they had any evidence of active infection, if their body weight exceeded 125% ideal body weight, if they had a Life expectancy of less than 12 weeks, or if they had a performance status z2 according to Southwest Group criteria for oncology patients.
Interventions
Intraosseous access device
An intraosseous access device (Osteoport, LifeQuest Medical, San Antonio, TX) was implanted in the iliac crest of all study subjects. The access device consisted of a port (reservoir) capped with a self-sealing silicon septum, through which medications could be given repeatedly (Fig. 1). The reservoir was connected to a hollow orthopedic screw (shaft) through which medications and fluids reached the intraosseous space. After implantation, the device was completely contained under the skin.
Implantation of the intraosseous access device
The intraosseous access device was implanted in the study subjects under either general or local anesthesia by surgeons trained in intraosseous implantation. A curvilinear incision was made adjacent to the anticipated site of the intraosseous access port (approximately 6 cm posterior to the anterior superior iliac spine). Guidewire pins were used
to define the planes of the inner and outer walls of the pelvis. A custom orthopedic drill bit, centered between the guide wires, was used to drill the hole for the intraosseous port. After irrigating the hole with saline, the self-tapping intraosseous port was gently inserted into the hole with a custom driver and then hand tightened to ensure proper seating within the bone (Figs. 2 and 3).
Placement of the port was confirmed by intraoperative x- ray. Patency of the port was confirmed by flushing the port with saline. The seal around the port was confirmed by direct observation after infusion of saline with Methylene blue dye. Subjects were evaluated 24 to 72 hours postoperatively and at biweekly intervals thereafter.
Accessing the intraosseous port
Before accessing the port, the skin surrounding the port site was prepared with an antiseptic cleanser. The silicon septum of the port was located by palpation. Once located, a noncoring needle or catheter attached to a clamped air-free extension tube was inserted into the port perpendicular to the septum. The extension tube was unclamped once the correct position of the needle or catheter was confirmed. Patency of the intraosseous access port was maintained by flushing after each use with preservative-free heparin.
Pharmacokinetic study protocol
Pharmacokinetic studies for each subject were conducted within 2 weeks of implantation of the intraosseous port. Subjects were randomized into 2 groups. The first group received a bolus of morphine sulfate through the intra- osseous access port, followed by a second bolus, intrave- nously, 24 hours later. The second group received the same procedures in reverse order.
Randomization of the order of morphine sulfate delivery routes was accomplished by each study site calling the central data center and receiving an assignment from the study nurse, who alternated the order of delivery routes as each patient was enrolled. The sequence was blinded to the

Fig. 1 Implantable intraosseous infusion device.
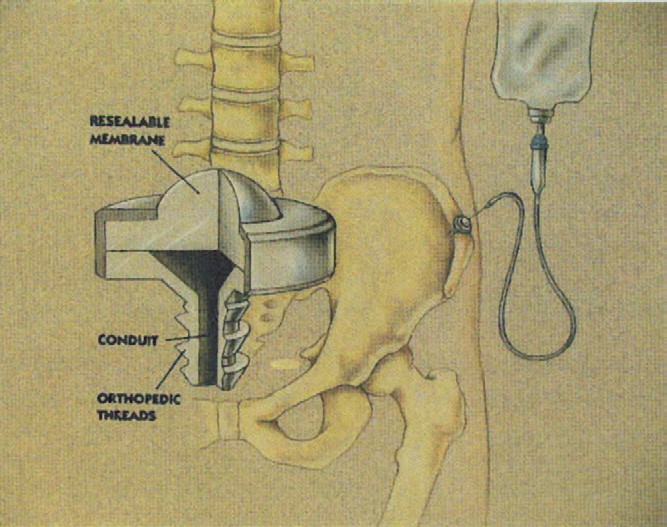
Fig. 2 Infusion through the device.
individual investigators at the study sites. Because of the nature of the study, neither the investigators nor the participants could be blinded as to the assignment of order of drug delivery routes.
The study dose consisted of a 5-mg bolus of morphine sulfate administered over a 15-second period, followed by 2 mL of normal saline infused over a 45-second period. Tubing lengths were identical for both intraosseous and
Standard pharmacokinetic parameters were then calculated from the data and the plots. For each subject, the maximum plasma concentration for morphine sulfate (Cmax) and its corresponding time (Tmax) were recorded for the subject’s observed plasma concentration-time profile following infu- sion by each delivery route.
Individual elimination rate (kel) values were determined by linear regression of the respective natural logarithm of morphine plasma concentrations vs time during the terminal phase. Either the final 5 or the final 6 points were used in the calculation of each kel value, which ever resulted in the regression line with the greater correlation coefficient. elimination half-life (t1/2) was calculated as 0.693/kel.
The AUC and the area under the first moment time curve (AUMC) were calculated by the linear trapezoidal rule up to the last measurable data point with extrapolation to infinity (l).
The apparent volume of distribution at steady state (Vdss) was calculated as
Vdss = Dose x AUMC(0-l)/AUC2 .
(0-l)
Plasma clearance (Clp) was calculated as: C1p = Dose/AUC(0-l).
The volume of distribution of the central compartment (Vd) was calculated as:
Serial blood samples (5 mL) were collected from a second intravenous line before dosing (baseline); at end of infusion (1
Vd = Dose/AUC
(0-l)
x kel.
minute); at 2, 5, 10, 15, 30, 45, 60, 90 minutes; and at 2, 3, 4, 6, and 8 hours postinfusion. Blood samples were collected into heparinized tubes and centrifuged at 2000 revolutions per minute for 15 minutes within 30 minutes of collection. The plasma was then removed and transferred to polyethylene tubes, which were labeled and stored at -208C until analysis. Upon completion of the pharmacokinetic study, patients were followed for up to 180 days to determine the long-term tolerance to the intraosseous access port. There were no
adverse events related to the pharmacokinetic study.
Methods of measurements
Morphine sulfate plasma concentrations
Morphine sulfate concentration for each plasma sample was determined by radioimmunoassay (COAT-ACOUNT, Diagnostic Products Co, Los Angeles, CA) at Maryland Medical Laboratory (Baltimore, MD). The range of the assay was 2.5 to 125 ng/mL. The sensitivity of the assay was 2.5 ng/mL, and the accuracy of the assay ranged from 93.4% to 97.8%.
Data collection, processing, and outcome measures
Pharmacokinetic calculations
For each subject, plots were constructed of plasma concentration of morphine sulfate vs sampling time.
Data analysis
Mean pharmacokinetic parameters for each administra- tion route were compared by analysis of variance for significant differences due to intraosseous or intravenous administration route, sequence of administration, and subject within sequence. For all analyses, effects were considered to be statistically significant if P V .05.
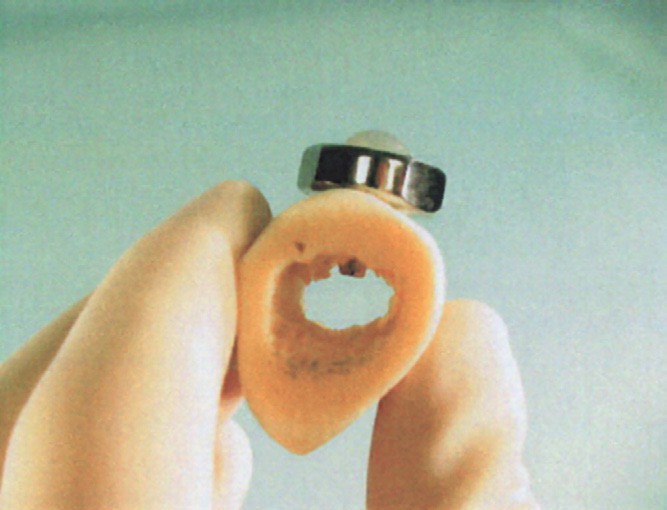
Fig. 3 Placement of the device in bone.
The primary objective of the study was to determine the systemic bioavailability of morphine sulfate administered by the intraosseous and intravenous route in the same patients. Power calculations were based on the AUC as the primary pharmacokinetic parameter. The study was designed to detect a 10% difference in the AUC means with 80% power using a 2-sided paired t test.
Mean ratios of intraosseous/intravenous were calculated for all pharmacokinetic parameters, along with 90% confidence intervals around the ratios. SAS version 6.07 software (SAS Institute, Cary, NC) software was used for the statistical analysis.
Results
Characteristics of study subjects
Eight clinical centers enrolled 25 subjects (9 men, 16 women) with a mean age of 57 years (range, 27-77 years).
All subjects had metastatic disease at the time of placement of the intraosseous port and had failed at least one attempt at conventional intravenous access. Primary cancer diagnoses included breast cancer (9 subjects), gastrointestinal cancer (5 subjects), Lung cancer (4 subjects), ovarian cancer (2 subjects), renal cancer (1 subject), leukemia (1 subject), multiple myeloma (1 subject), osteosarcoma (1 subject), and chondrosarcoma (1 subject).
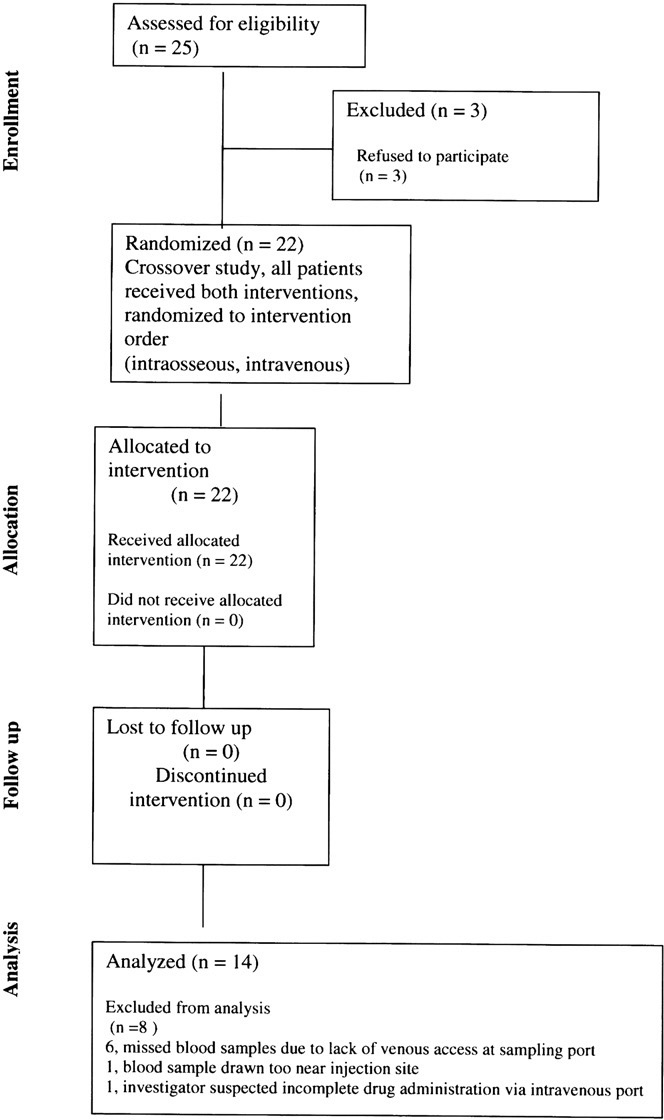
The flow of subjects through the study is shown in Fig. 4. Three subjects declined to participate in the pharmacoki- netic study. Pharmacokinetic data were obtained from 22 subjects. Full sets of evaluable pharmacokinetic data were available for 14 subjects. For the remaining 8 subjects, missed blood samples occurred at or near the expected maximum morphine concentration due to lack of venous access at the sampling port (6 subjects), blood samples drawn too near the injection site (1 subject), or because the investigator suspected incomplete administration of mor- phine via the intraosseous port (1 subject).
Main results
Mean plasma morphine sulfate concentrations vs sam- pling time are displayed in Fig. 5. The results of the analysis of variance revealed no significant differences between the intraosseous and intravenous route of administration on the plasma morphine concentration vs sampling time data. Likewise, there were no significant differences between the
2 administration routes in many of the pharmacokinetic parameters, including AUC0-t, AUC0-l, Cmax, Tmax, Cl, Vdss, kel, and t1/2, indicating that intraosseous and intrave- nous delivery routes for morphine were essentially equiv- alent (Table 1). There was, however, a statistically
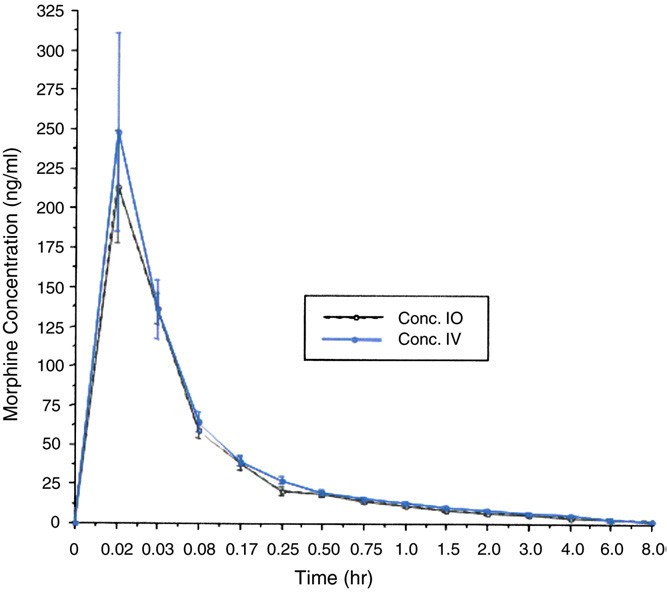
Fig. 5 Plasma concentration (mean F SEM) vs time curve (0- 8 hours) of morphine sulfate in 14 subjects after a 5 mg bolus of morphine sulfate administered intraosseously (dashed line) or intravenously (solid line).
significant difference in the volume of distribution in the central compartment, Vd ( P = .0247), which in the opinion of the investigators was thought to be due to a minor deposition effect near the intraosseous port or in the bone marrow. There were no significant differences due to sequence of administration or subject within sequence.
Comparison of the intraosseous/intravenous ratios of the mean values (Table 1) indicates that the peak concentration (Cmax) for morphine was 19% less than delivered by the intravenous route. However, the AUC0-l intraosseous/intravenous ratio indicates that intraosseous administration only resulted in a 1.0% lower exposure to morphine than by the intravenous route. The untrans- formed 90% confidence interval for the ratio of Cmax was 54% to 109%. The 90% confidence intervals for the ratios of AUC0-t and AUC0-l were 76% to 104% and 81% to 116%, respectively.
Limitations
The major limitation of this study is that the data evaluated only morphine sulfate and may not apply to other classes of drugs. A second limitation is the exclusion of 8 patients due to a lack of complete data sets, although this limitation is not uncommon among pharmacokinetic studies in subjects with such Severe comorbidities, such as cancer. Despite this, the statistical analysis reached the required power level to attain statistically valid conclusions regarding the bioequivalence of morphine administration among the 2 delivery routes. A further limitation is that although the investigators were interested in the pharma- cokinetics of intraosseously administered medications in emergency medicine, it was not possible to conduct a study in healthy volunteers or emergency patients using intraosseous devices designed for emergency use.
Discussion
When emergency professionals give drugs or fluids to patients in life-threatening situations, they need to know that
the route of administration can deliver clinically effective doses to target organs in reasonable times. Many emergency patients are in a state of shock where the body’s natural defenses shut down peripheral circulation. This makes it difficult or impossible to access peripheral veins for delivery of life-saving drugs and fluids.
In such cases, practitioners have looked for alternate routes of drug administration such as intramuscular, intranasal, subcutaneous, sublingual, rectal, and endotrache- al. Although all these routes can be effective in certain circumstances, few offer the speed and bioavailability of the intravenous route. Two alternate vascular access routes that have gained popularity recently are the Central venous lines and intraosseous vascular access.
Although intraosseous infusion of drugs and fluids for adults is rapidly becoming the standard of care in the emergency medical community [3], the scientific question remains-bDoes the intraosseous route of administration equal the intravenous route for such indications?Q Dye and radioactive tracer studies indicate that drugs given intra- osseously enter the central circulation within seconds, comparable to peripheral intravenous lines [17]. In addition, studies have shown the pharmacological effects of drugs, such as epinephrine on heart rate and blood pressure, are equivalent when given via either route [4,18]. Several pharmacokinetic studies in animals indicate that the intra- osseous route is equivalent to the intravenous route for a wide variety of medications [10-16].
To date, there has been only one other published study on the pharmacokinetics of intraosseously administered drugs in humans; that study compared the venous concentrations of lidocaine administered via intraosseous injection or by maxillary infiltration injection in dental patients. The investigators found that the 2 drug administration routes resulted in identical plasma concentrations of lidocaine for each of the 9 time points sampled [19].
The answer to the question, bDoes intraosseous equal intravenous?Q required a prospective, randomized, multi- center trial in human subjects. Therefore, we conducted this trial comparing the intraosseous route to the intravenous
route of drug administration to provide the rigorous scientific data necessary for statistical significance. Data from the present study suggest that intraosseous infusion of medications is a viable alternative and pharmacokinetically equivalent route for morphine administration in human patients with difficult venous access. Because the basic mechanics, anatomy, and physiology of intraosseous infu- sion are ostensibly the same across patient populations, the results of the study should readily extrapolate to emergency patients. The study was conducted with patients with cancer; however, aside from their disease, the patients were relatively healthy, ambulatory patients with good vital signs. The intraosseous device provided access to the intraosseous space to study the pharmacokinetics of intraosseously administered medications. Although the basic anatomy and physiology of intraosseous infusion should be substan- tially equivalent across most types of medications and fluids for patients with intact circulatory status, the authors caution that the study results may not be generally applicable across all Medication classes, intraosseous Anatomical sites, and patient populations.
Because emergency medical personnel are mostly focused on effect and time to effect for fast-acting medications in emergency situations, the most relevant pharmacokinetic parameters from the current study are Cmax (peak concentration) and Tmax (time to peak concentration). There were no significant differences in these 2 parameters between the intraosseous and intravenous routes of mor- phine administration, indicating similar bioavailability between the 2 routes.
Other emergency medications have a longer duration of effect. In the current study, a similar pattern was observed in the short- and long term (8-hour study period) pharmaco- kinetics of intraosseously administered morphine sulfate, as evidenced by the equivalent intraosseous/intravenous ratios for AUC. The intraosseous/intravenous ratios of mean AUC values were 0.90 (90%) for AUC(0-t) and 0.99 (99%) for AUC(0-l). The 90% confidence interval for the ratio of AUC(0-l) (81%-116%) falls within the 80% to 120% criterion generally recognized as an acceptable level for comparison of the pharmacokinetics of different routes of administration of medication.
The plasma concentration vs sampling time curves for both routes of administration were similar, as were nearly all the pharmacokinetic parameters. There was a statisti- cally significant difference in Vd, the volume of distribution in the central compartment. It is the opinion of the investigators that this could have been due to a deposition effect near the intraosseous access port or in the bone marrow. However, there was no significant difference in Vdss, the apparent volume of distribution at steady state, suggesting that the amount of residual morphine near the intraosseous port or in the bone marrow was minimal.
Although the current study was conducted on patients with cancer, the study has obvious implications for intra-
osseous infusion of medications in emergency medicine. Because most emergency medications are expected to reach target organs or exert their effect systematically within minutes, the pharmacokinetic equivalency of intraosseous and intravenous administration of morphine sulfate (partic- ularly at the earlier sampling times, ie, at 2, 5, 10, 15, and 20 minutes) reinforces the applicability of intraosseous delivery for emergency medicine. The results of the study indicate that there was no significant delay in morphine sulfate reaching the systemic circulation via the intraosseous route. In addition, morphine sulfate is frequently used in emer- gency medicine for treatment of myocardial infarction, congestive heart failure, and pain control.
In summary, this pharmacokinetic study supports the bioequivalence of intraosseous and intravenous administra- tion of morphine sulfate in human subjects. Data are limited to morphine sulfate and may not apply to other classes of drugs. However, with the more widespread use of intra- osseous drug delivery in emergency medicine, there is great impetus to validate the Pharmacokinetic profiles of other classes of commonly used emergency medications.
References
- Dubick MA, Holcomb JB. A review of intraosseous access: current status and military application. Mil Med 2000;165:552 - 8.
- Hazinski MF, editor. Pediatric life support (PALS) provider manual. Dallas (Tex)7 American Heart Association; 2002.
- ACLS Guidelines. Part 7.2 management of cardiac arrest. Circulation 2005;112:IV-170 - 171.
- Orlowski JP, Porembka DT, Gallagher JM, Lockrem JD, VanLente F. Comparison study of intraosseous, central intravenous, and peripheral intravenous infusions of emergency drugs. Am J Dis Child 1990;144:112 - 7.
- Orlowski JP. Emergency alternatives to intravenous access: intra- osseous, intratracheal, and other-site drug administration. Pediatr Clin North Am 1994;41:1183 - 99.
- LaRocco BG, Wang HE. Intraosseous infusion. Prehosp Emerg Care 2003;7:280 - 5.
- Brunette DD, Fischer R. Intravascular access in Pediatric cardiac arrest. Am J Emerg Med 1988;6:577 - 9.
- Rosetti VA, Thompson BM, Miller J, Mateer JR, Aprahamian C. Intraosseous infusion: an alternative route of pediatric intravascular access. Ann Emerg Med 1985;14:885 - 8.
- Lavis M. Pre-hospital adult intraosseous infusion. Pre-hosp Immed Care 1999;3:89 - 92.
- Jaimovich DG, Snabino CL, Ringer TV, Peters GR. Comparison of intraosseous and intravenous routes of anticonvulsant administration in a porcine model. Ann Acad Emerg Med 1989;18:842 - 6.
- Jaimovich DG, Kumar A, Francom S. Evaluation of intraosseous vs. intravenous antibiotic levels in a porcine model. AJDC 1991;145: 946 - 9.
- Brickman KR, Rega P, Guiness M. A comparative study of intraosseous versus peripheral intravenous infusion of diazepam and phenobarbital in dogs. Ann Acad Emerg Med 1987;16:1441 - 4.
- Cameron JL, Fontanarosa PB, Passalaqua AM. A comparative study of peripheral to central circulation delivery times between intraosseous and intravenous injection using a radionucleotide technique in normovolemic and hypovolemic canines. J Emerg Med 1989;7:123 - 7.
- Goldstein R, Lavy E, Shem-Tov M, Glickman A, Bark H, Ziv G. Pharmacokinetics of ampicillin administered intravenously and intra- osseously to kittens. Res Vet Sci 1995;59:186 - 7.
- Chastagner P, Lozniewski A, Lascombes P, Barberi-Heyob M, Methers PM, Merlin JL. Pharmacokinetic longitudinal studies of antibiotics administered via a permanent intraosseous device in micropigs. Med Pediatr Oncol 2001;36:635 - 40.
- Golenz MR, Wilson WD, Carlson GP, Craychee TJ, Mihalyi JE, Knox L. Effect of route of administration and age on the pharmacokinetics of amikacin administered by the intravenous and intraosseous routes to 3 and 5-day-old foals. Equine Vet J 1994;26: 367 - 73.
- Hoskins S, Nascimento P, Espana J, Kramer G. Pharmacokinetics of intraosseous drug delivery during CPR. Shock 2005;23:35.
- Sapien R, Stein H, Padbury JF, Thio S, Hodge D. Intraosseous versus intravenous epinephrine infusions in lambs: pharmacokinetics and pharmacodynamics. Pediatr Emerg Care 1992;8:179 - 83.
- Wood M, Reader A, Nusstein J, Beck M, Padgett D, Weaver J. Comparison of intraosseous and infiltration injections for venous lidocaine Blood concentrations and heart rate changes after injection of 2% lidocaine with 1:100,000 epinephrine. J Endod 2005;43:5 - 8.
