Clinical triage decision vs risk scores in predicting the need for endotherapy in upper gastrointestinal bleeding
American Journal of Emergency Medicine (2012) 30, 129-134

Original Contribution
Clinical triage decision vs risk scores in predicting the need for endotherapy in Upper gastrointestinal bleeding?,??
Farees T. Farooq MD a,b, Michael H. Lee MD b, Ananya Das MD c,
Rahul Dixit MD b, Richard C.K. Wong MBBS b,?
aGastro One, Memphis, TN 38138, USA
bDivision of Gastroenterology and Liver Disease, university hospitals Case Medical Center, Cleveland, OH 44106-5066, USA cDivision of Gastroenterology, Mayo Clinic, Scottsdale, AZ 85259, USA
Received 13 April 2010; revised 3 November 2010; accepted 4 November 2010
Abstract
Background: Acute Upper gastrointestinal hemorrhage (UGIH) is a common reason for hospitalization with substantial associated morbidity, mortality, and cost. Differentiation of high- and low-risk patients using established Risk scoring systems has been advocated.
The aim of this study was to determine whether these scoring systems are more accurate than an emergency physician’s clinical decision making in predicting the need for endoscopic intervention in acute UGIH.
Methods: Patients presenting to a tertiary care medical center with acute UGIH from 2003 to 2006 were identified from the hospital database, and their clinical data were abstracted. One hundred ninety-five patients met the inclusion criteria and were included in the analysis. The clinical Rockall score and Blatchford score (BS) were calculated and compared with the clinical triage decision (intensive care unit vs non-intensive care unit admission) in predicting the need for endoscopic therapy.
Results: Clinical Rockall score greater than 0 and BS greater than 0 were sensitive predictors of the need for endoscopic therapy (95% and 100%) but were poorly specific (9% and 4%), with overall accuracies of 41% and 39%. At higher score cutoffs, clinical Rockall score greater than 2 and BS greater than 5 remained sensitive (84% and 87%) and were more specific (29% and 33%), with overall accuracies of 48% and 52%. Clinical triage decision, as a surrogate for predicting the need for endoscopic therapy, was moderately sensitive (67%) and specific (75%), with an overall accuracy (73%) that exceeded both risk scores.
Conclusions: The clinical use of risk scoring systems in acute UGIH may not be as good as clinical decision making by emergency physicians.
(C) 2012
? Presented at an oral session at Digestive Disease Week 2007, Washington, DC, and published in abstract form (Gastrointest Endosc 2007;65:AB122.)
?? No authors have any conflicts of interest to disclose. There are no
competing interests to disclose.
* Corresponding author.
E-mail address: [email protected] (R.C.K. Wong).
Introduction
Acute upper gastrointestinal hemorrhage (UGIH) is a common clinical problem accounting for 250000 hospitaliza- tions annually in the United States alone [1-3]. The frequency and severity of this problem and its Associated costs impose a significant burden on limited Health care resources. As with
0735-6757/$ - see front matter (C) 2012 doi:10.1016/j.ajem.2010.11.007

Rockall scoring system: scores for age, shock, and comorbidity comprise the cRS. Scores for diagnosis and major stigmata of recent hemorrhage + cRS equals the complete Rockall score. SBP indicates systolic blood pressure; P, pulse rate; CHF, congestive heart failure; CAD, coronary artery disease; MW, Mallory-Weiss; UGI, upper GI; SRH, stigmata of recent hemorrhage.
Renal or Liver failure and cancer
CHF, CAD, and other major diseases
UGI cancer
Fresh blood, adherent clot, visible or spurting vessel
All other
MW tear, no lesion None, flat spot
Diagnosis Major SRH
Comorbidity None
Age (y) Shock
3
2
>=80
SBP b100 mm Hg
1
60-79
SBP >=100 mm Hg, P >=100 beats/min
Table 1 Rockall score
Score 0
b60
SBP >=100 mm Hg, P b100 beats/min
other common disease processes, strategies to optimize patient outcome while minimizing health care resource use are desirable. This task can be accomplished by triaging patients presenting with acute UGIH to appropriate Levels of care.
Although urgent endoscopy in all patients with acute UGIH and subsequent endoscopy-based triage may reduce downstream costs and length of hospitalization, this is not always a feasible option [4]. As such, Triage systems that can differentiate high- and low-risk patients at initial presentation before endoscopy may be more practical. A variety of risk scores both for UGIH and lower gastrointestinal (GI) hemorrhage have been developed based on clinical and endoscopic variables encountered in patients with acute GI bleeding. The goals of these tools are to identify patients most likely to suffer an adverse outcome and, therefore, more likely to benefit from early, aggressive management as well as to identify those most likely to have a benign course so that early hospital discharge or even outpatient management can be considered.
The Rockall score is a scoring system that was developed to predict mortality in patients with acute UGIH [5-8]. The score consists of a clinical component composed of preen- doscopy variables as well as an endoscopic component (Table 1). The scores derived from the Rockall scoring system-the clinical Rockall score (cRS) and the complete Rockall score-have been investigated and validated in several studies [8-10]. Recurrent bleeding, studied as a secondary outcome in the study of Rockall et al [7], was also found to be a strong predictor of mortality and was analyzed separately but not included in the formal risk score calculation. A follow-up study by Rockall et al found that the Rockall score could be successfully applied not only to predict mortality but also to identify patients at low risk for recurrent bleeding who may be appropriate for outpatient management or early hospital discharge. The Blatchford score (BS) was subsequently developed and prospectively validated to address the, perhaps, more clinically applicable outcome of the need for clinical intervention to control bleeding, defined as the need for blood transfusion and endoscopic or surgical intervention [11]. Unlike the Rockall
score, which includes endoscopic variables, the BS is based only on clinical variables that are available before endoscopy (Table 2). Since its original description in 1996, the Rockall score has been extensively used in research studies as a benchmark of severity when comparing different study populations and as a means for identifying low-risk patients who may be amenable to lower levels of care.
Despite the published evidence for the applicability of upper GI bleed risk scores, physicians have been reluctant to incorporate these scores into clinical practice for a variety of reasons including the perception that score calculation is difficult and time consuming and the belief that calculated scores cannot substitute for the intuition and decision making of an experienced clinician. For the same reasons, the use of
Table 2 Blatchford score
|
Admission risk marker Score
|
|
BUN (mmol/L) 6.5-8.0 2 8.0-10.0 3 10.0-25.0 4 N25 6 Hgb (g/L), male Hgb (g/L), female 120-130 100-120 1 100-120 3 b100 b100 6 Systolic blood pressure (mm Hg) 100-109 1 90-99 2 b90 3 Other markers Pulse >=100 beats/min 1 Melena 1 Syncope 2 Liver disease 2 |
|
Blatchford score: note that unit conversion is necessary before score calculation (BUN typically reported as milligrams per deciliter, and hemoglobin level as grams per deciliter in the United States). BUN indicates blood urea nitrogen; Hgb, hemoglobin. |
other triage tools and clinical care paths is frequently dismissed as the practice of “cookbook medicine.”
At our institution and most others, the initial major triage decision in patients presenting with acute UGIH is made by emergency physicians. These emergency physicians use their specialized training and expertise to individually assess the severity of bleeding and to initiate prompt resuscitation, where appropriate. In general, patients who are deemed the most severe are triaged for admission to the intensive care unit (ICU) vs less severe patients who are triaged for admission to a non-ICU ward. The aim of this study was to compare decision making by emergency physicians (clinical triage) vs decision making by the use of established UGIH risk scoring systems in predicting the need for endoscopic therapy.
Methods
This was a retrospective, single-center study based at University Hospitals Case Medical Center in Cleveland, OH, a 947-bed tertiary care medical center that houses a 24-hour, level 2 trauma emergency department (ED). In the ED, there are 15 full-time staff physicians, 11 of whom are board- certified emergency physicians along with 3 internists and 1 Family Medicine specialist. The study was approved by the hospital’s institutional review board for human investigation. Inclusion criteria were as follows: all adults 18 years or older presenting to the ED with acute UGIH between July 2003 and June 2006. Acute UGIH was defined as observed hematemesis, melena, or NG aspirate containing gross or altered blood or recent history of hematemesis or melena. Exclusion criteria were transferees from outside hospitals, patients with secondary UGIH (patients who developed UGIH after presentation/admission for an unrelated complaint), and
patients ultimately diagnosed with lower GI hemorrhage.
Patients were identified from the hospital’s computerized database using International Classification of Diseases, Ninth Revision (ICD-9), codes validated for UGIH in previous studies [12]. These included esophageal varices with bleeding (ICD-9 Clinical Modification 456.0); Mallory- Weiss syndrome (530.7); gastric ulcer with hemorrhage (531.00, 531.01, 531.20, 531.21, 531.40, 531.41, 531.60,
531.61); Duodenal ulcer with hemorrhage (532.00, 532.01,
532.20, 532.21, 532.40, 532.41, 532.60, 532.61); peptic
ulcer, site unspecified, with hemorrhage (533.00, 533.01, 533.20, 533.21, 533.40, 533.41, 533.60, 533.61); gastro-
jejunal ulcer with hemorrhage (534.00, 534.01, 534.20,
534.21, 534.40, 534.41); gastritis with hemorrhage (535.01,
535.11, 535.21, 535.31, 535.41, 535.51); duodenitis with
hemorrhage (535.61); angiodysplasia of the stomach/duode- num with hemorrhage (537.83); and hematemesis (578.0). Individual medical records of the identified patients were reviewed to select patients who met the inclusion criteria.
The following data from identified patients were then recorded: age, sex, pertinent medical history (congestive heart failure, coronary artery disease, renal failure, liver
failure/cirrhosis, malignancy, and chronic obstructive pul- monary disease), vitals signs, and examination findings on presentation, laboratory studies (blood urea nitrogen and hemoglobin level), admission location (ICU vs general medical ward), and endoscopic findings and endoscopic therapy. The need for endoscopic therapy was defined as injection of saline, epinephrine, or sclerosants and applica- tion of clips, bands, or thermal therapy (heat probe, bipolar electrocoagulation, and argon plasma coagulation) as well as bleeding that resulted in death or required emergency surgery, Transjugular intrahepatic portosystemic shunt , or balloon tamponade, where endoscopic therapy was not feasible or technically possible.
Clinical Rockall score and BS were calculated for each patient. The clinical triage decision (CTD) of ICU vs non- ICU admission was used as a surrogate marker for prediction of the need for endoscopic therapy. Clinical Rockall score greater than 0 and BS greater than 0 were used as thresholds for predicting the need for endoscopic therapy. Test characteristics (sensitivity, specificity, accuracy, positive predictive value [PPV], and negative predictive value [NPV]) for CTD, cRS, and BS were calculated using standard 2 x 2 tables. This analysis was repeated using thresholds of cRS greater than 2 and BS greater than 5. A 10% random sample of patients was rechecked to ensure the integrity of data collection and score calculation.
Results
A total of 1244 patients were identified from the search of the hospital database by ICD-9 codes, as previously described. Of these, 195 patients met the inclusion criteria and were included in the study. The mean age was 63.8 years. All 195 patients (100%) were admitted to the hospital. No patients presenting to the ED with UGIH were discharged home from the ED. Of the 195 admitted patients, 78 patients (40%) were admitted to the ICU, and 117 patients (60%) were admitted to the general medical ward. Of the admitted patients, 194 (99.5%) underwent esophagogastroduodeno- scopy (EGD). One patient with severe Variceal hemorrhage died before EGD. Of the 195 patients in the study, 64 (33%) needed endoscopic therapy. Sixty percent of patients admitted to the ICU required endoscopic therapy. In contrast, only 18% of patients admitted to the medical ward required endoscopic therapy. There were a total of 8 inhospital deaths (4.1% mortality). The distribution of causes of UGIH in the study patients is shown in Fig. 1. The mean cRS was 3.5, and the mean BS was 8.8.
Clinical Rockall score and BS were compared with the CTD in predicting the need for endoscopic therapy. The test characteristics for each modality are shown in Table 3. Clinical Rockall score greater than 0 and BS greater than 0 were both highly sensitive (95% and 100%) but poorly specific (9% and 4%) in predicting the need for endoscopic therapy. In contrast, CTD was both moderately sensitive and
15%
Mallory- Weiss tear 8%
AVMs 4%
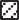 Other 29%
Other 29%
disease 44%
frequently used risk stratification systems in critical care [14]. Similarly, in hepatology, the Child-Pugh score, Maddrey discriminant function, and Model for end-stage liver disease score often form the foundation for subsequent clinical decision making [15,16]. In the case of Model for End-Stage Liver Disease, the risk score alone typically supersedes the clinician’s assessment of how sick the patient is and how soon liver transplantation should be undertaken. With only slight improvements in mortality rates associated with acute UGIH over the past several decades, accurately identifying patients most likely to benefit from aggressive management at the time of initial presentation remains a clinically important endeavor. However, despite the recognition of risk scoring systems for
Fig. 1 Causes of upper GI bleeding in the study population. AVM indicates arteriovenous malformation.
specific (67% and 75%) in predicting the need for endoscopic therapy. The overall accuracy of CTD (73%) was superior to that of cRS greater than 0 (41%) and BS greater than 0 (39%). The impact of liberalizing the score thresholds for cRS and BS in predicting the need for endoscopic therapy was examined. Based on prior studies identifying cRS greater than 2 and BS greater than 5 as thresholds beyond which patients were more likely to require intervention, the test characteristics of cRS and BS using these cutoffs were also calculated [11]. For cRS greater than 2 and BS greater than 5, there were slight decreases in sensitivity (84% and 87%), with modest increases in specificity (29% and 33%). Nonetheless, the overall accuracy of CTD remained substantially higher (73%) than the accuracy of the risk scores with 48% and 52%, respectively, for cRS greater than 2 and BS greater than 5. Similarly, CTD had the highest PPV (60%), with the lowest being cRS greater than 0 (37%). Blatchford score greater than 0 had the highest NPV (100%), with the remainder (cRS N0, cRS N2, BS N5, and CTD) having similar NPVs (77%-82%). Only 3% of patients had BS of 0.
Discussion
Risk stratification tools are commonly used in medical practice. For instance, the criteria of Ranson et al [13] and Acute Physiology and Chronic Health Evaluation scores are
Table 3 Performance of risk scores and physicians’ CTD in predicting the need for endoscopic therapy
UGIH, they have not been widely used in clinical practice. In part, this may be due to the perception that the clinical decision making of an experienced physician is superior to decision making based on a mathematical formula.
Efforts to identify other predictive factors of adverse outcomes in UGIH continue to be prevalent in the medical literature [17-19]. In a recent study from an Italian Registry of patients with nonvariceal UGIH, Marmo et al [17] found that several factors included in both the Rockall score and BS were independent predictors of mortality, specifically advanced age, low hemoglobin level at presentation, and significant comorbidities. Emergency physicians have also examined the potential benefits of risk scoring systems in the management of patients with acute UGIH. In a 2007 study from Taiwan, Chen et al [9] found the BS to be highly sensitive in predicting the need for clinical intervention, but in this study, both the cRS and BS had poor specificities (25% and 38%, respectively), a factor that may limit the efficacy of these scores as practical triage instruments.
In this study, we demonstrated that, in our patient population, the triage decision of ED physicians to ICU vs non-ICU beds was an accurate predictor of the need for endoscopic therapy and that the CTD was more accurate than both cRS and BS, even when the score thresholds were modified. This is a particularly interesting finding given that it is contrary to what one might expect. Both risk scoring systems have been validated in several diverse patient populations, although neither score has been formally studied in a prospective fashion in which Clinical decisions regarding timing of endoscopy or triage have been made based on scores alone. However, it is commonly presumed
|
Accuracy (%) |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
||
|
Clinical Rockall N0
|
92 |
41 |
95 |
9 |
37 (30-45) |
80 (51-95) |
|
Clinical Rockall N2 |
75 |
48 |
84 |
29 |
39 (31-48) |
77 (62-87) |
|
Blatchford N0 |
97 |
39 |
100 |
4 |
37 (30-44) |
100 (48-100) |
|
Blatchford N5 |
74 |
52 |
87 |
33 |
42 (34-50) |
82 (69-91) |
|
CTD |
73 |
67 |
75 |
60 (48-71) |
81 (72-87) |
|
|
cRS indicates clinical Rockall score; BS, Blatchford score; CTD, clinical triage decision. Test characteristics of cRS, BS, and CTD are shown. Ninety-five percent of confidence intervals for PPV and NPV are indicated in parentheses. |
||||||
that these scores are likely to be useful in making real-time clinical decisions. The strength of the initial studies that gave rise to these risk scores may be one reason for this. In addition, it is likely that endoscopists underappreciate the clinical intuition of experienced emergency physicians. Although emergency physicians may not be as familiar as gastroenterologists with Forrest classification and other endoscopic stigmata of recent hemorrhage, as demonstrated in this study, they are very good at differentiating how “sick” patients with UGIH are and in appropriately triaging the most severe patients to the ICU where urgent EGD and endoscopic therapy can be performed. One of the proposed advantages of the Rockall score and BS has been that nonendoscopist physicians might benefit from incorporating these scores into their practice as a triage tools or as guides for determining early discharge. We did not examine the use of these scores in the discharge process for low-risk patients; however, there does not appear to be a clear benefit for emergency physicians to use these scores above and beyond their own clinical judgment at the time of initial triage for high-risk patients, at least at our institution.
There are some limitations to the study. First, CTD (ICU vs non-ICU admission) was used as a surrogate marker for predicting the need for endoscopic therapy. This has not previously been used as a surrogate marker for these purposes. However, ours is the first study to compare clinical decision making with calculated risk scores. We felt that the CTD was the most practical surrogate to use in this retrospective study. If the study were conducted prospec- tively, one would directly ask emergency physicians to predict the need for endoscopic therapy rather than use a surrogate, which would probably be more accurate. None- theless, because specific knowledge of endoscopic stigmata of recent hemorrhage and the need for endoscopic therapy may not be widely known by emergency physicians, we felt that CTD was a more practical surrogate for assessment of risk from the standpoint of the emergency physician. Second, this study presumes that the CTD of the emergency physician is based solely on clinical decision making. Certainly, other factors such as bed availability and recommendations from consulting physicians may play a role in the triage decision, but it is likely that clinical factors including age, comorbid- ities, and suspicion for major active or recent bleeding are the primary contributors to CTD. In addition, it should be noted that prediction of the need for endoscopic therapy was not the original intent of the cRS, although numerous studies addressing the Rockall score have extrapolated its use for outcomes other than mortality. Another limitation is that the need for blood transfusion was not included as a component of the need for endoscopic therapy in this study and may have contributed to the poor specificity of the BS in which blood transfusion was considered a “clinical intervention.” Because the need for transfusion may be subjective and based more so on the clinical scenario rather than acuity or severity of bleeding, the need for transfusion was not included in measuring the need for endoscopic therapy. For
example, a stable patient with subacute bleeding and markedly low hemoglobin level may receive a transfusion, whereas a patient with variceal hemorrhage may not receive a transfusion if the hemoglobin level is within reasonable range. In these instances, the need for transfusion is not equivalent to the need for endoscopic therapy. Finally, our conclusions about CTD vs the risk scores are largely based on the calculated overall accuracy, which represents a weighted average of the sensitivity and specificity. Although accuracy may be somewhat misleading in some cases, with a prevalence of 33% of the need for endoscopic therapy in our study population, the use of overall accuracy as a summary test characteristic seems reasonable.
It should also be emphasized that the findings of this single-center study may not be applicable to other medical institutions, to other emergency physicians, and to other patient populations. For instance, Oei et al [20] have specifically studied hospitalization of low-risk patients with acute UGIH at a tertiary care and community hospital and have found that health care resource use was significantly greater at the community hospital, with 71% of low-risk patients admitted to ICU or monitored beds, whereas only 49% were triaged to the same level of care at a neighboring tertiary care hospital.
In conclusion, this study suggests that the clinical use of risk scoring systems in acute UGIH may not be as good as clinical decision making by emergency physicians. If risk scoring systems are ever going to be widely accepted and used by physicians, prospective studies are needed at both tertiary care and community hospitals to directly compare physician decision making with decisions made by these scoring systems.
References
- Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1995;90:206-10.
- Gilbert DA. Epidemiology of upper gastrointestinal bleeding. Gastrointest Endosc 1990;36(Suppl):S8-S13.
- Barkun A, Bardou M, Marshall JK, for the Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003;139:843-57.
- Lee JG, Turnipseed S, Romano PS, Vigil H, Azari R, Melnikoff N, Hsu R, Kirk D, Sokolove P, Leung JW. Endoscopy-based triAge SIgnificantly reduces hospitalization rates and costs of treating upper GI bleeding: a randomized controlled trial. Gastrointest Endosc 1999;50:755-61.
- Rockall TA, Logan RFA, Devlin HB, Northfield TC. Variation in outcome after acute gastrointestinal haemorrhage. Lancet 1995;346: 346-50.
- Rockall TA, Logan RFA, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut 1996;38:316-21.
- Rockall TA, Logan RFA, Devlin HB, Northfield TC. Selection of patient for early discharge or outpatient care after acute upper gastrointestinal haemorrhage. Lancet 1996;347:1138-40.
- Rockall TA, Logan RFA, Devlin HB, Northfield TC. Influencing the practice and outcome in acute upper gastrointestinal haemorrhage. Gut 1997;41:606-11.
- Chen IC, Hung MS, Chiu TF, Chen JC, Hsiao CT. Risk scoring systems to predict need for clinical intervention for patients with nonvariceal upper gastrointestinal tract bleeding. Am J Emerg Med 2007;25:774-9.
- Gralnek IM, Dulai GS. Incremental value of Upper endoscopy for triage of patients with acute non-variceal upper-GI hemorrhage. Gastrointest Endosc 2004;60:9-14.
- Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper gastrointestinal haemorrhage. Lancet 2000;356: 1318-21.
- Cooper GS, Chak A, Lloyd LE, Yurchick PJ, Harper DL, Rosenthal GE. The accuracy of diagnosis and procedural codes for patients with upper GI hemorrhage. Gastrointest Endosc 2000;51: 423-6.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Localio SA. Objective early identification of Severe acute pancreatitis. Am J Gastroenterol 1974;61:443-51.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a Severity of disease classification system. Crit Care Med 1985;13: 818-29.
- Maddrey WC, Boitnott JK, Bedine MS, Weber Jr FL, Mezey E, White Jr RI. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75:193-9.
- Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end- stage liver disease. Hepatology 2001;33:464-70.
- Marmo R, Koch M, Cipolletta L, et al. Predictive factors of mortality from nonvariceal upper gastrointestinal hemorrhage: a multicenter study. Am J Gastroenterol 2008;103:1639-47.
- Romagnuolo J, Barkun AN, Enns R, Armstrong D, Gregor J. Simple Clinical predictors may obviate urgent endoscopy in selected patients with nonvariceal upper gastrointestinal tract bleeding. Arch Intern Med 2007;167:265-70.
- Imperiale TF, Dominitz JA, Provenzale DT, et al. Predicting poor outcome from acute gastrointestinal hemorrhage. Arch Intern Med 2007;167:1291-6.
- Oei TT, Dulai GS, Gralnek IM, Chang D, Kilbourne AM, Sale GA. Hospital care for low-risk patients with acute, nonvariceal upper GI hemorrhage: a comparison of neighboring community and Tertiary care centers. Am J Gastroenterol 2002;97:2271-8.



