An electrocardiogram technician improves in-hospital first medical contact-to-electrocardiogram times: a cluster randomized controlled interventional trial☆☆☆★
Affiliations
- Department of Emergency Medicine, Medical University of Vienna, Waehringerguertel 18-20/6D, A-1090 Vienna, Austria
- Vienna Municipal Ambulance Service, Radetzkystrasse 1, A-1030 Vienna
Affiliations
- Department of Emergency Medicine, Medical University of Vienna, Waehringerguertel 18-20/6D, A-1090 Vienna, Austria
Affiliations
- Department of Emergency Medicine, Medical University of Vienna, Waehringerguertel 18-20/6D, A-1090 Vienna, Austria
Affiliations
- Department of Emergency Medicine, Medical University of Vienna, Waehringerguertel 18-20/6D, A-1090 Vienna, Austria
Affiliations
- Department of Emergency Medicine, Medical University of Vienna, Waehringerguertel 18-20/6D, A-1090 Vienna, Austria
Affiliations
- Department of Emergency Medicine, Medical University of Vienna, Waehringerguertel 18-20/6D, A-1090 Vienna, Austria
Correspondence
- Corresponding author. Tel.: + 43 1 40400 1964; fax: + 43 1 40400 1965.

Affiliations
- Department of Emergency Medicine, Medical University of Vienna, Waehringerguertel 18-20/6D, A-1090 Vienna, Austria
Correspondence
- Corresponding author. Tel.: + 43 1 40400 1964; fax: + 43 1 40400 1965.
Affiliations
- Department of Emergency Medicine, Medical University of Vienna, Waehringerguertel 18-20/6D, A-1090 Vienna, Austria
 Article Info
Article Info
To view the full text, please login as a subscribed user or purchase a subscription. Click here to view the full text on ScienceDirect.
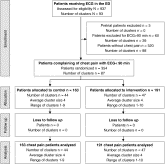
Fig. 1
Flowchart showing eligible patients.
Abstract
Background
In the case of chest pain, the current guidelines require electrocardiogram (ECG) recording and patient assessment within 10 minutes upon arrival in the emergency department.
Methods
We investigated the effect of an ECG technician (ECG-T) on in-hospital first medical contact-to-ECG times (iFMC-to-ECG) investigated in a cluster randomized, controlled trial. Allocation of intervention was concealed. Staff satisfaction and feasibility was defined as a secondary outcome. Delays between ECG and the availability of an emergency physician and the assessment of ECG were additionally evaluated.
Results
A total of 163 (44 clusters) and 191 (47 clusters) patients were allocated to control and intervention, respectively. Twenty-seven (17%) of 163 patients in the control group vs 110 (58%) of 191 patients in the intervention group received ECG registration within 10 minutes (risk ratio, 3.40 [2.24-5.15]; P < .001). The iFMC-to-ECG time was 23 (95% confidence interval [CI], 20-27) minutes for the control group vs 9 (95% CI, 8-11) minutes for the intervention group (P < .001). Nursing staff judged the feasibility of intervention with a median of 1 (interquartile range [IQR], 1-1 (on a scale of 1 [best] to 5 [worst]), perceived workload alleviation with a median of 1 (IQR, 1-1), and improvement of quality of care with a median of 1 (IQR, 1-2). The ECG-to-EP time was 78 (95% CI, 64-92) seconds, and diagnosis was made within 17 (95% CI, 16-18) seconds.
Conclusions
Delays of iFMC-to-ECG can be effectively addressed by implementation of an ECG-T. The service of an ECG-T is feasible and improves staff satisfaction. Both ECG-to-EP time and ECG assessment constitute no relevant delay.
Abbreviations:
ECG (electrocardiogram), ED (emergency department), STEMI (ST-segment elevation myocardial infarction), NSTEMI (non–ST-segment elevation myocardial infarction), DTB (door-to-balloon), PPCI (primary percutaneous coronary intervention), ECG-T (ECG technician), iFMC (in-hospital first medical contact), iFMC-to-ECG (in-hospital first medical contact-to-ECG), RN (resident nurse), EP (emergency physician), ECG-to-EP (electrocardiogram-to-emergency physician), CI (confidence interval), RR (risk ratio), IQR (interquartile range)To access this article, please choose from the options below
Purchase access to this article
Claim Access
If you are a current subscriber with Society Membership or an Account Number, claim your access now.
Subscribe to this title
Purchase a subscription to gain access to this and all other articles in this journal.
Institutional Access
Visit ScienceDirect to see if you have access via your institution.
☆Trial registry: http://www.controlled-trials.com/; identifier: ISRCTN68587870.
☆☆Conflict of interest: None to declare.
★Authors' contributions: R.v.T., D.R., and H.H. designed the study. C.W., B.H., W.S., and C.H. critically revised the study protocol. R.v.T., D.R., C.W., and B.H. participated in data acquisition. All authors participated in analysis and interpretation of the data. R.v.T. drafted the manuscript. D.R., C.W., B.H., H.H., W.S., and C.H. critically revised the manuscript for important intellectual content. H.H. provided statistical expertise. W.S. and C.H. supervised the whole study. All authors read and approved the final manuscript.
Related Articles
Searching for related articles..


