Prospective, randomized, double-blind controlled trial comparing vapocoolant spray vs placebo spray in adults undergoing venipuncture☆☆☆★★★
Correspondence
- Cleveland Clinic, Emergency Services Institute, 9500 Euclid Ave, E- 19, Cleveland, Ohio 44195. Tel.: +1 216 445 4598, +1 216 444 1748 (administrative assistant, Mimi); fax: 1 216 445 4552.

Correspondence
- Cleveland Clinic, Emergency Services Institute, 9500 Euclid Ave, E- 19, Cleveland, Ohio 44195. Tel.: +1 216 445 4598, +1 216 444 1748 (administrative assistant, Mimi); fax: 1 216 445 4552.
 Article Info
Article Info
To view the full text, please login as a subscribed user or purchase a subscription. Click here to view the full text on ScienceDirect.
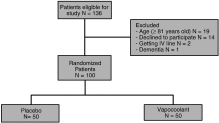
Fig. 1
Participant flow diagram according to the Consolidated Standards of Reporting Trial guidelines.
Fig. 2
The pain of venipuncture on NRS: box and whisker plots of NRS for the placebo spray and the vapocoolant spray. The median is the center line, the boxes are the 25th to 75th percentile, and the triangle is the mean.
Abstract
Introduction
Topical anesthetics are used to decrease procedural pain such as venipuncture. Advantages of vapocoolants include rapid onset, ease of application, low cost, and lack of associated pain of injection and other needlestick-related risks. We hypothesized that the pain of venipuncture would be reduced by at least 1.8 points on a 10-point numerical rating scale after application of a vapocoolant compared with placebo.
Methods
We conducted a prospective, randomized, double-blind controlled trial of vapocoolant vs placebo spray in 100 adults (ages 18-80) requiring venipuncture in a hospital emergency department or observation unit. The primary efficacy outcome was the difference in pain scores immediately after venipuncture, measured on a 10-point verbal numeric rating scale from 0 (none) to worst (10). Safety outcomes included local adverse effects (edema, erythema, blanching) and changes in vital signs (VS).
Results
Patient characteristics and venipuncture procedure were not significantly different for the 2 groups. The median (interquartile range) pain of venipuncture was 3 (1.2-5) in the placebo group and 1 (0-3) in the vapocoolant group, P < .001. Skin checklist revealed the following: vapocoolant—minimal blanching 4%, minimal erythema 18% which resolved within 5 minutes; placebo—no visible skin changes. Photographs at 5 to 10 minutes revealed no visible skin changes in any patient. There were 2 complaints: “very wet and cold on skin” (placebo) and “felt burning on skin” (vapocoolant).
Conclusion
The vapocoolant significantly decreased venipuncture pain in adults compared with placebo and was well tolerated with minor adverse effects that resolved quickly. There were no significant differences in VS and no visible skin changes documented at the site by photographs taken within 5 to 10 minutes postspray/venipuncture.
To access this article, please choose from the options below
Purchase access to this article
Claim Access
If you are a current subscriber with Society Membership or an Account Number, claim your access now.
Subscribe to this title
Purchase a subscription to gain access to this and all other articles in this journal.
Institutional Access
Visit ScienceDirect to see if you have access via your institution.
☆Presentations: American Society of Regional Anesthesia and Pain, November 2012, Miami, FL; Society of Academic Emergency Medicine, May 2012, Atlanta, GA; American College of Emergency Physicians Scientific Assembly, October 2011, San Francisco, CA.
☆☆Supported by a grant from the Gebauer Company.
★The funding source had no involvement in study design; collection, analysis, and interpretation of the data; writing of the report; and the decision to submit the article for publication.
★★The trial is registered at ClinicalTrials.gov NCT01712776.
Related Articles
Searching for related articles..