Usefulness of new and traditional serum biomarkers in children with suspected appendicitis☆☆☆☆☆☆
Affiliations
- Pediatric Emergency Department, Cruces University Hospital, Bilbao, Basque Country, Spain
Correspondence
- Corresponding author at: Pediatric Emergency Department, Department of Pediatrics, Cruces University Hospital, Plaza de Cruces s/n, E-48903 Barakaldo, Bizkaia, Spain. Tel.: +34 94 6006388; fax: +34 94 6006076.

Affiliations
- Pediatric Emergency Department, Cruces University Hospital, Bilbao, Basque Country, Spain
Correspondence
- Corresponding author at: Pediatric Emergency Department, Department of Pediatrics, Cruces University Hospital, Plaza de Cruces s/n, E-48903 Barakaldo, Bizkaia, Spain. Tel.: +34 94 6006388; fax: +34 94 6006076.
Affiliations
- Pediatric Emergency Department, Cruces University Hospital, Bilbao, Basque Country, Spain
Affiliations
- Pediatric Surgery Department, Cruces University Hospital, Bilbao, Basque Country, Spain
Affiliations
- Pediatric Emergency Department, Cruces University Hospital, Bilbao, Basque Country, Spain
Affiliations
- Laboratory Department, Cruces University Hospital, Bilbao, Basque Country, Spain
Affiliations
- Epidemiologic Unit, Cruces University Hospital, Bilbao, Basque Country, Spain
 Article Info
Article Info
To view the full text, please login as a subscribed user or purchase a subscription. Click here to view the full text on ScienceDirect.
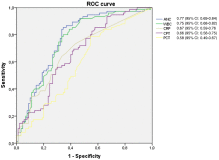
Fig. 1
Receiver operating characteristic curves for separately biomarkers.
Fig. 2
Receiver operating characteristic curve: multivariate model (AUC, 0.78; 95% CI, 0.71-0.84).
Abstract
Objective
The objective of the study is to evaluate the usefulness of the leukocyte (white blood count [WBC]) and neutrophil (absolute neutrophil count [ANC]) counts; the values of C-reactive protein (CRP), procalcitonin, and calprotectin (CP); and the APPY1 Test panel of biomarkers, to identify children with abdominal pain at low risk for appendicitis.
Method
Children 2 to 14 years of age with abdominal pain suggesting acute appendicitis (AA) were prospectively included. Procalcitonin, calprotectin, C-reactive protein, white blood count, ANC, and the new plasma APPY1 Test were performed. The final diagnosis was determined by histopathology in cases of AA and telephone follow-up in children discharged without AA.
Results
Between February 2012 and June 2013, 185 children were enrolled with an average age of 9.32 ± 2.7 years. Eighty-nine (48.1%) were finally diagnosed with AA. The APPY1 Test panel showed the highest discriminatory power, sensitivity of 97.8 (95% confidence interval [CI], 92.2-99.4), negative predictive value of 95.1 (95% CI, 83.9-98.7), negative likelihood ratio of 0.06 (95% CI, 0.01-0.22), and specificity of 40.6 (95% CI, 31.3-50.5). A negative APPY1 Test and ANC less than 7500 per milliliter provided a sensitivity of 100 (95% CI, 95.9-100), negative predictive value of 100 (95% CI, 89.8-100), and specificity of 35.4 (95% CI, 26.6-45.4). In the multivariate analysis, only the APPY1 Test and ANC greater than 7500 per milliliter were significant risk factors for AA (odds ratio, 13.76; 95% CI, 3.02-62.57, and odds ratio, 6.37; 95% CI, 2.89-14.28, respectively).
Conclusions
The APPY1 Test panel with ANC could be useful in identifying children with abdominal pain suggestive of AA who are at low risk for this disease.
To access this article, please choose from the options below
Purchase access to this article
Claim Access
If you are a current subscriber with Society Membership or an Account Number, claim your access now.
Subscribe to this title
Purchase a subscription to gain access to this and all other articles in this journal.
Institutional Access
Visit ScienceDirect to see if you have access via your institution.
☆Funding source: No external funding was secured for this study.
☆☆Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
☆☆☆Conflict of interest: The authors have no conflicts of interest to disclose.
Related Articles
Searching for related articles..