Prospective Validation of a Biomarker Panel to Identify Pediatric Emergency Department Patients with Abdominal Pain Who Are at Low Risk of Acute Appendicitis
Affiliations
- Division of Emergency Medicine, Newton-Wellesley Hospital, Newton, MA
Correspondence
- Corresponding author at: Department of Emergency Medicine, Newton-Wellesley Hospital, 2014 Washington St., Newton, MA, 02462.

Affiliations
- Division of Emergency Medicine, Newton-Wellesley Hospital, Newton, MA
Correspondence
- Corresponding author at: Department of Emergency Medicine, Newton-Wellesley Hospital, 2014 Washington St., Newton, MA, 02462.
Affiliations
- Emory University, Children's Healthcare of Atlanta, Atlanta, GA
Affiliations
- Seton University of Texas Southwestern Clinical Research Institute, University Medical Center at Brackenridge, Austin, TX
- Dell Children's Medical Center, Austin, TX
Affiliations
- Scottish Rite Hospital, Children's Healthcare of Atlanta, Atlanta, GA

 Article Info
Article Info
To view the full text, please login as a subscribed user or purchase a subscription. Click here to view the full text on ScienceDirect.
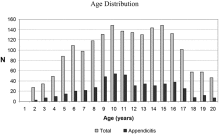
Fig. 1
Age Distribution
Fig. 2
Patient Disposition
AA+ = acute appendicitis, AA- = no acute appendicitis, LTFU = Lost to follow up.
*captured on return visit or follow up
Fig. 3
Diagnosis and Disposition by Biomarker Results
AA+ = acute appendicitis, AA- = no acute appendicitis, *Includes those diagnosed as AA+ but treated with antibiotics only, **Captured on return visit or follow up, +one negative appendectomy on follow up
Abstract
Objectives
To prospectively validate the diagnostic accuracy of a biomarker panel consisting of WBC, CRP, and MRP 8/14 levels in identifying pediatric patients with abdominal pain who are at low risk of appendicitis.
Methods
This prospective observational study enrolled subjects aged 2-20 years presenting to 29 U.S. emergency departments with abdominal pain suggesting possible acute appendicitis. Blood samples were analyzed for WBC, CRP, and MRP 8/14 levels from which the composite biomarker panel results were calculated, then correlated with the final diagnosis either positive or negative for acute appendicitis.
Results
2201 patients were enrolled, with 1887 completing all aspects of the study. Prevalence of appendicitis in this cohort was 25.3%. The biomarker panel exhibited a sensitivity of 97.1% (95% CI, 95.1-98.2%), negative predictive value of 97.4% (95% CI, 95.8-98.5%), negative likelihood ratio of 0.08 (95% CI, 0.05-0.13), with a specificity of 37.9% (95% CI, 35.4-40.4%) for appendicitis. The panel correctly identified 534 of 1410 (37.8%) patients who did not have appendicitis with 14 (2.9%) false negatives. Overall, 23.7% (132/557) of CT scans were done for patients with negative biomarker panel results, including 31.2% (131/420) of patients who had CT but did not have appendicitis.
Conclusion
This biomarker panel exhibited high sensitivity and negative predictive value for acute appendicitis in this large prospective cohort. This panel may be useful in identifying pediatric patients who are at low risk of appendicitis and might be followed clinically, potentially reducing the dependence on CT in the evaluation for acute appendicitis.
To access this article, please choose from the options below
Purchase access to this article
Claim Access
If you are a current subscriber with Society Membership or an Account Number, claim your access now.
Subscribe to this title
Purchase a subscription to gain access to this and all other articles in this journal.
Institutional Access
Visit ScienceDirect to see if you have access via your institution.
Funding: This research was funded by Venaxis, Inc., Castle Rock, CO
Prior Presentations: ACEP 2014, Chicago, IL, October 27, 2014
Conflict of Interest Disclosure: David S. Huckins, MD - paid consulting fees by Venaxis Inc. for design, execution, and analysis of study results, and as member of Clinical Study Steering Committee and Chair of Publication Committee; reimbursed expenses for travel to FDA meetings and conferences.
Harold K. Simon, MD, MBA - served on the Venaxis Clinical Study Steering Committee and as a sponsored trial site investigator.
Karen Copeland, PhD - paid consulting fees by Venaxis Inc. as member of Clinical Study Steering Committee and for study design and analysis of study results.
Truman J. Milling, Jr. MD - paid consulting fees by Venaxis Inc. as Chair of Clinical Study Steering Committee.
Philip R. Spandorfer, MD - sponsored trial site investigator.
Halim Hennes, MD - sponsored trial site investigator.
Coburn Allen, MD - sponsored trial site investigator.
Joseph Gogain, PhD - employed by Venaxis, Inc.
Related Articles
Searching for related articles..


