Abstract
Introduction
Objective
Discussion
Conclusion
Keywords
1. Introduction
World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov). Published January 30, 2020. Accessed February 18, 2020.
World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Published March 11, 2020. Accessed March 20, 2020.
World Health Organization. WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Published February 11, 2020. Accessed March 08, 2020.
World Health Organization. Q&A on coronaviruses. https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. Published February 11, 2020. Accessed March 08, 2020.
New images of Novel Coronavirus SARS-CoV-2 now available|NIH: National Institute of Allergy and Infectious Diseases. https://www.niaid.nih.gov/news-events/novel-coronavirus-sarscov2-images. Accessed March 18, 2020.
CDC. 2019 ovel Coronavirus (2019-nCoV) frequently asked questions and answers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/faq.html. Published February 11, 2020. Accessed March 18, 2020.
Naming the coronavirus disease (COVID-2019) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. Accessed March 12, 2020.
CDC. HAN Archive - 00426|Health Alert Network (HAN). https://emergency.cdc.gov/han/han00426.asp. Published February 11, 2020. Accessed March 19, 2020.
- News Scan for Dec 31
Taylor DB. A timeline of the coronavirus. The New York Times. https://www.nytimes.com/2020/02/13/world/coronavirus-timeline.html. Published February 13, 2020. Accessed March 18, 2020.
- News Scan for Dec 31
Salcedo A, Cherelus G. Coronavirus travel restrictions, across the globe. The New York Times. https://www.nytimes.com/article/coronavirus-travel-restrictions.html. Published March 20, 2020. Accessed March 20, 2020.
- CNN JG
- AW
CDC. 2019 Novel Coronavirus (2019-nCoV) situation summary. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/summary.html. Published February 11, 2020. Accessed March 18, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) prevention & treatment. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/prevention-treatment.html. Published February 11, 2020. Accessed March 19, 2020.
Identify, Isolate, Inform: Emergency Department Evaluation and Management for Patients Under Investigation (PUIs) for Ebola Virus Disease (EVD)|Emergency Services|Clinicians|Ebola (Ebola Virus Disease)|CDC. https://www.cdc.gov/vhf/ebola/clinicians/emergency-services/emergency-departments.html. Published August 30, 2019. Accessed February 18, 2020.
2. Discussion
2.1 Virology
New images of Novel Coronavirus SARS-CoV-2 now available|NIH: National Institute of Allergy and Infectious Diseases. https://www.niaid.nih.gov/news-events/novel-coronavirus-sarscov2-images. Accessed March 18, 2020.
Coronavirus|Human Coronavirus Types|CDC. https://www.cdc.gov/coronavirus/types.html. Published February 16, 2020. Accessed March 12, 2020.
New images of Novel Coronavirus SARS-CoV-2 now available|NIH: National Institute of Allergy and Infectious Diseases. https://www.niaid.nih.gov/news-events/novel-coronavirus-sarscov2-images. Accessed March 18, 2020.
CDC. 2019 ovel Coronavirus (2019-nCoV) frequently asked questions and answers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/faq.html. Published February 11, 2020. Accessed March 18, 2020.
CDC. HAN Archive - 00426|Health Alert Network (HAN). https://emergency.cdc.gov/han/han00426.asp. Published February 11, 2020. Accessed March 19, 2020.
Coronavirus|Human Coronavirus Types|CDC. https://www.cdc.gov/coronavirus/types.html. Published February 16, 2020. Accessed March 12, 2020.
CDC. 2019 ovel Coronavirus (2019-nCoV) frequently asked questions and answers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/faq.html. Published February 11, 2020. Accessed March 18, 2020.
CDC. HAN Archive - 00426|Health Alert Network (HAN). https://emergency.cdc.gov/han/han00426.asp. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) situation summary. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/summary.html. Published February 11, 2020. Accessed March 18, 2020.
CDC. 2019 ovel Coronavirus (2019-nCoV) frequently asked questions and answers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/faq.html. Published February 11, 2020. Accessed March 18, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) situation summary. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/summary.html. Published February 11, 2020. Accessed March 18, 2020.
- Li Q.
- Guan X.
- Wu P.
- et al.
CDC. Coronavirus Disease 2019 (COVID-19): animals and Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/prepare/animals.html. Published March 16, 2020. Accessed March 20, 2020.
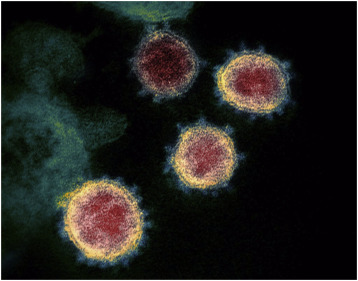
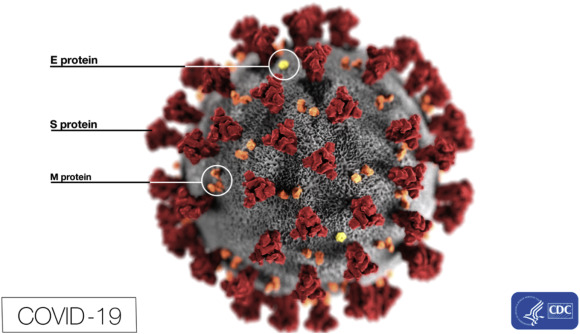
2.2 Epidemiology
CDC. HAN Archive - 00426|Health Alert Network (HAN). https://emergency.cdc.gov/han/han00426.asp. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) situation summary. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/summary.html. Published February 11, 2020. Accessed March 18, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) transmission. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html. Published February 11, 2020. Accessed March 12, 2020.
- WHO
World Health Organization. Coronavirus disease 2019 (COVID-19) situation report – 60. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200320-sitrep-60-covid-19.pdf?sfvrsn=8894045a_2. Published March 20, 2020. Accessed March 20, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) cases in the U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-in-us.html. Published March 20, 2020. Accessed March 20, 2020.
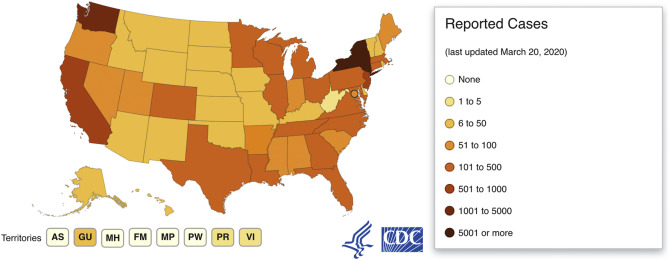
World Health Organization. Q&A on coronaviruses. https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. Published February 11, 2020. Accessed March 08, 2020.
CDC. HAN Archive - 00426|Health Alert Network (HAN). https://emergency.cdc.gov/han/han00426.asp. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) situation summary. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/summary.html. Published February 11, 2020. Accessed March 18, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) transmission. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html. Published February 11, 2020. Accessed March 12, 2020.
Coronavirus Disease 2019 Transcript for CDC Media Telebriefing. Centers for Disease Control and Prevention. https://wwwdev.cdc.gov/media/releases/2020/s0215-Diamond-Princess-Repatriation.html. Published February 18, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) situation summary. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/summary.html. Published February 11, 2020. Accessed March 18, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) transmission. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html. Published February 11, 2020. Accessed March 12, 2020.
Coronavirus Disease 2019 Transcript for CDC Media Telebriefing. Centers for Disease Control and Prevention. https://wwwdev.cdc.gov/media/releases/2020/s0215-Diamond-Princess-Repatriation.html. Published February 18, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) transmission. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html. Published February 11, 2020. Accessed March 12, 2020.
Coronavirus Disease 2019 Transcript for CDC Media Telebriefing. Centers for Disease Control and Prevention. https://wwwdev.cdc.gov/media/releases/2020/s0215-Diamond-Princess-Repatriation.html. Published February 18, 2020. Accessed March 19, 2020.
- Zou L.
- Ruan F.
- Huang M.
- et al.
- Bai Y.
- Yao L.
- Wei T.
- et al.
- Wax R.S.
- Christian M.D.
CDC. 2019 Novel Coronavirus (2019-nCoV) transmission. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html. Published February 11, 2020. Accessed March 12, 2020.
- Li Q.
- Guan X.
- Wu P.
- et al.
Rettner R. How does the new coronavirus compare with the flu? livescience.com. https://www.livescience.com/new-coronavirus-compare-with-flu.html. Published March 19, 2020. Accessed March 11, 2020.
- Li Q.
- Guan X.
- Wu P.
- et al.
- WHO
- Szabo L.
Parker E, The Vaccine Centre, London School of Hygiene & Tropical Medicine. Covid 2019 tracker. https://vac-lshtm.shinyapps.io/ncov_tracker/. Published 2020. Accessed March 21, 2020.
Parker E, The Vaccine Centre, London School of Hygiene & Tropical Medicine. Covid 2019 tracker. https://vac-lshtm.shinyapps.io/ncov_tracker/. Published 2020. Accessed March 21, 2020.
Parker E, The Vaccine Centre, London School of Hygiene & Tropical Medicine. Covid 2019 tracker. https://vac-lshtm.shinyapps.io/ncov_tracker/. Published 2020. Accessed March 21, 2020.
World Health Organization. Q&A on coronaviruses. https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. Published February 11, 2020. Accessed March 08, 2020.
| Disease | Case fatality rate | Deaths | Cases |
|---|---|---|---|
| COVID-19 (2019) | 4.2 | 11,299 | 272,166 |
| Influenza (2017) | N/A | 145,000 | 54,481,000 |
| Ebola (2014) | 39.53 | 11,323 | 28,646 |
| H1N1 (2009) | 0.1 | 18,449 | 60,800,000 |
| SARS (2003) | 9.56 | 774 | 8096 |
World Health Organization. Q&A on coronaviruses. https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. Published February 11, 2020. Accessed March 08, 2020.
CDC. 2019 ovel Coronavirus (2019-nCoV) frequently asked questions and answers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/faq.html. Published February 11, 2020. Accessed March 18, 2020.
World Health Organization. Q&A on coronaviruses. https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. Published February 11, 2020. Accessed March 08, 2020.
- Li Q.
- Guan X.
- Wu P.
- et al.
CDC. 2019 Novel Coronavirus (2019-nCoV) symptoms. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html. Published February 11, 2020. Accessed March 19, 2020.
- Wang M.
- Wu Q.
- Xu W.
- et al.
Shah N. Higher co-infection rates in COVID19. Medium. https://medium.com/@nigam/higher-co-infection-rates-in-covid19-b24965088333. Published March 19, 2020. Accessed March 20, 2020.
2.3 Special populations
CDC. 2019 Novel Coronavirus (2019-nCoV) clinical care. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) pregnant women. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/pregnancy-faq.html. Published February 11, 2020. Accessed March 19, 2020.
- Qiao J.
CDC. 2019 Novel Coronavirus (2019-nCoV) pregnant women. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/pregnancy-faq.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) pregnant women. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/pregnancy-faq.html. Published February 11, 2020. Accessed March 19, 2020.
- Qiao J.
CDC - Health Care Workers, Infectious Agents - NIOSH workplace safety and health topic. https://www.cdc.gov/niosh/topics/healthcare/infectious.html. Published November 6, 2018. Accessed March 12, 2020.
- Lietz J.
- Westermann C.
- Nienhaus A.
- Schablon A.
American College of Emergency Physicians. Two emergency physicians in critical condition. ACEP. https://www.acep.org/corona/covid-19-articles/a-statement-from-acep-president-william-jaquis-md-facep/. Published March 14, 2020. Accessed March 21, 2020.
Italy has a world-class health system. The coronavirus has pushed it to the breaking point. NBC News. https://www.nbcnews.com/health/health-news/italy-has-world-class-health-system-coronavirus-has-pushed-it-n1162786. Accessed March 20, 2020.
2.4 Clinical presentation
CDC. 2019 ovel Coronavirus (2019-nCoV) frequently asked questions and answers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/faq.html. Published February 11, 2020. Accessed March 18, 2020.
CDC. HAN Archive - 00426|Health Alert Network (HAN). https://emergency.cdc.gov/han/han00426.asp. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) symptoms. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) flowchart for healthcare professionals. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/identify-assess-flowchart.html. Published February 11, 2020. Accessed March 19, 2020.
- Guan W.
- Ni Z.
- Hu Y.
- et al.
- Pan L.
- Mu M.
- Ren H.G.
- et al.
- Pan L.
- Mu M.
- Ren H.G.
- et al.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Infection Prevention and Control Recommendations. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html. Published February 11, 2020. Accessed March 20, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) healthcare infection prevention and control FAQs for COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-prevention-control-faq.html. Published February 11, 2020. Accessed March 19, 2020.
2.5 Initial approach to COVID-19 in the emergency department
CDC. Coronavirus Disease 2019 (COVID-19): evaluating and testing persons for Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published February 11, 2020. Accessed March 21, 2020.
| Suspected case A. A patient with acute respiratory illness (fever and at least one sign/symptom of respiratory disease, e.g., cough, shortness of breath), AND a history of travel to or residence in a location reporting community transmission of COVID-19 disease during the 14 days prior to symptom onset; OR B. A patient with any acute respiratory illness AND having been in contact with a confirmed or probable COVID-19 case (see definition of contact) in the last 14 days prior to symptom onset; OR C. A patient with severe acute respiratory illness (fever and at least one sign/symptom of respiratory disease, e.g., cough, shortness of breath; AND requiring hospitalization) AND in the absence of an alternative diagnosis that fully explains the clinical presentation. |
| Probable case A. A suspect case for whom testing for the COVID-19 virus is inconclusive. OR B. A suspect case for whom testing could not be performed for any reason. |
| Confirmed case A person with laboratory confirmation of COVID-19 infection, irrespective of clinical signs and symptoms. See laboratory guidance for details: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technicalguidance/laboratory-guidance |
| Contact A contact is a person who experienced any one of the following exposures during the 2 days before and the 14 days after the onset of symptoms of a probable or confirmed case:
|
CDC. 2019 Novel Coronavirus (2019-nCoV) evaluating and reporting PUI. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published February 11, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) evaluating and reporting PUI. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published February 11, 2020. Accessed March 19, 2020.
| 1. Hospitalized patients who have signs and symptoms compatible with COVID-19 in order to inform decisions related to infection control. 2. Other symptomatic individuals such as, older adults and individuals with chronic medical conditions and/or an immunocompromised state that may put them at higher risk for poor outcomes (e.g., diabetes, heart disease, receiving immunosuppressive medications, chronic lung disease, chronic kidney disease). 3. Any persons including healthcare personnela, who within 14 days of symptom onset had close contactb with a suspect or laboratory-confirmedc COVID-19 patient, or who have a history of travel from affected geographic areasd within 14 days of their symptom onset. |
| Notes: aFor healthcare personnel, testing may be considered if there has been exposure to a person with suspected COVID-19 without laboratory confirmation. Because of their often extensive and close contact with vulnerable patients in healthcare settings, even mild signs and symptoms (e.g., sore throat) of COVID-19 should be evaluated among potentially exposed healthcare personnel. Additional information is available in CDC’s Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease 2019 (COVID-19). bClose contact is defined as— a) being within approximately 6 feet (2 meters) of a COVID-19 case for a prolonged period of time; close contact can occur while caring for, living with, visiting, or sharing a healthcare waiting area or room with a COVID-19 case – or – b) having direct contact with infectious secretions of a COVID-19 case (e.g., being coughed on) If such contact occurs while not wearing recommended personal protective equipment or PPE (e.g., gowns, gloves, NIOSH-certified disposable N95 respirator, eye protection), criteria for PUI consideration are met. cDocumentation of laboratory-confirmation of COVID-19 may not be possible for travelers or persons caring for COVID-19 patients in other countries. dAffected areas are defined as geographic regions where sustained community transmission has been identified. For a list of relevant affected areas, see CDC’s Coronavirus Disease 2019 Information for Travel. |
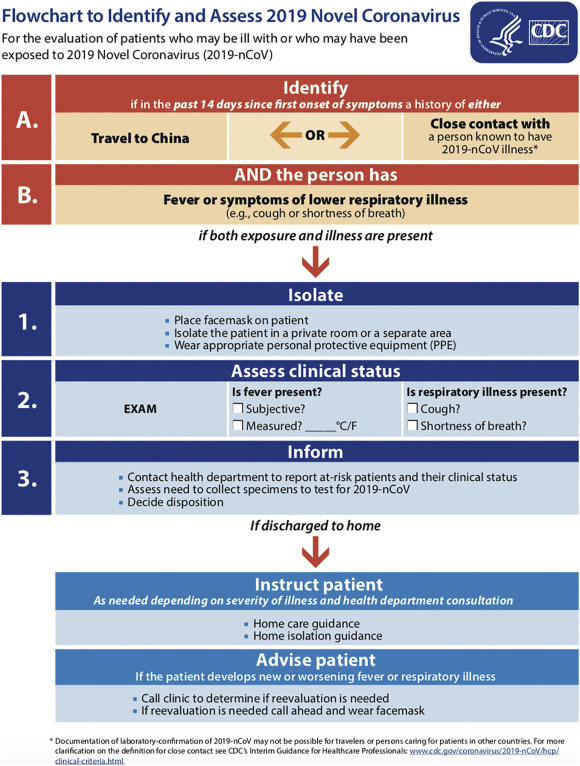
2.5.1 Pre-hospital setting
CDC. Coronavirus Disease 2019 (COVID-19) Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Published February 11, 2020. Accessed March 14, 2020.
- Wax R.S.
- Christian M.D.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Published February 11, 2020. Accessed March 14, 2020.
- Wax R.S.
- Christian M.D.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Published February 11, 2020. Accessed March 14, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Published February 11, 2020. Accessed March 14, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Published February 11, 2020. Accessed March 14, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Published February 11, 2020. Accessed March 14, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Published February 11, 2020. Accessed March 14, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Published February 11, 2020. Accessed March 14, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Published February 11, 2020. Accessed March 14, 2020.
2.5.2 Emergency department setting
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) infection control. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published February 11, 2020. Accessed March 19, 2020.
- Wax R.S.
- Christian M.D.
CDC. 2019 Novel Coronavirus (2019-nCoV) healthcare infection prevention and control FAQs for COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-prevention-control-faq.html. Published February 11, 2020. Accessed March 19, 2020.
- Wax R.S.
- Christian M.D.
CDC. 2019 Novel Coronavirus (2019-nCoV) infection control. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published February 11, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) evaluating and reporting PUI. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) infection control. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) infection control. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published February 11, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) infection control. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) infection control. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. HAN Archive - 00426|Health Alert Network (HAN). https://emergency.cdc.gov/han/han00426.asp. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) flowchart for healthcare professionals. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/identify-assess-flowchart.html. Published February 11, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) healthcare infection prevention and control FAQs for COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-prevention-control-faq.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) infection control. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published February 11, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) infection control. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) infection control. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) healthcare infection prevention and control FAQs for COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-prevention-control-faq.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) healthcare infection prevention and control FAQs for COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-prevention-control-faq.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) healthcare infection prevention and control FAQs for COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-prevention-control-faq.html. Published February 11, 2020. Accessed March 19, 2020.
Public Health Agency of Canada. Infection prevention and control for coronavirus disease (COVID-19): interim guidance for acute healthcare settings. aem. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/interim-guidance-acute-healthcare-settings.html#a4.18. Published February 24, 2020. Accessed February 27, 2020.
Public Health Agency of Canada. Infection prevention and control for coronavirus disease (COVID-19): interim guidance for acute healthcare settings. aem. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/interim-guidance-acute-healthcare-settings.html#a4.18. Published February 24, 2020. Accessed February 27, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Infection Prevention and Control Recommendations. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html. Published February 11, 2020. Accessed March 20, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Infection Prevention and Control Recommendations. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html. Published February 11, 2020. Accessed March 20, 2020.
Public Health Agency of Canada. Infection prevention and control for coronavirus disease (COVID-19): interim guidance for acute healthcare settings. aem. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/interim-guidance-acute-healthcare-settings.html#a4.18. Published February 24, 2020. Accessed February 27, 2020.
2.5.3 Considerations for airway management
- Wax R.S.
- Christian M.D.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
- Wax R.S.
- Christian M.D.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
- Wax R.S.
- Christian M.D.
- Wax R.S.
- Christian M.D.
- Wax R.S.
- Christian M.D.
- Wax R.S.
- Christian M.D.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
- Wax R.S.
- Christian M.D.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
- Wax R.S.
- Christian M.D.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
- Wax R.S.
- Christian M.D.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
2.6 Laboratory and radiographic findings
CDC. 2019 Novel Coronavirus (2019-nCoV) Testing. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) information for laboratories COVID-19 requests for diagnostic panels and virus. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/lab/tool-virus-requests.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) interim guidelines for collecting, handling, and testing clinical specimens from persons under investigation (PUIs) for Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Published February 11, 2020. Accessed March 20, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) interim guidelines for collecting, handling, and testing clinical specimens from persons under investigation (PUIs) for Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Published February 11, 2020. Accessed March 20, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) interim guidelines for collecting, handling, and testing clinical specimens from persons under investigation (PUIs) for Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Published February 11, 2020. Accessed March 20, 2020.
- Wang W.
- Xu Y.
- Gao R.
- et al.
Coronavirus Disease 2019 Transcript for CDC Media Telebriefing. Centers for Disease Control and Prevention. https://wwwdev.cdc.gov/media/releases/2020/s0215-Diamond-Princess-Repatriation.html. Published February 18, 2020. Accessed March 19, 2020.
CDC. 2019 ovel Coronavirus (2019-nCoV) frequently asked questions and answers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/faq.html. Published February 11, 2020. Accessed March 18, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) evaluating and reporting PUI. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published February 11, 2020. Accessed March 19, 2020.
2.7 Treatment
World Health Organization. Q&A on coronaviruses. https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. Published February 11, 2020. Accessed March 08, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) prevention & treatment. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/prevention-treatment.html. Published February 11, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
World Health Organization. DRAFT landscape of COVID-19 candidate vaccines. World Health Organization. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf?ua=1. Published March 20, 2020. Accessed March 21, 2020.
World Health Organization. Q&A on coronaviruses. https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. Published February 11, 2020. Accessed March 08, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) prevention & treatment. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/prevention-treatment.html. Published February 11, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
- Episode 38 - COVID-19 Update
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
World Health Organization. Q&A on coronaviruses. https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. Published February 11, 2020. Accessed March 08, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) clinical care. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Published February 11, 2020. Accessed March 19, 2020.
- Gordon C.J.
- Tchesnokov E.P.
- Feng J.Y.
- Porter D.P.
- Gotte M.
- Martinez M.A.
- Cao B.
- Wang Y.
- Wen D.
- et al.
- Gautret P.
- Lagier J.-C.
- Parola P.
- et al.
- Gautret P.
- Lagier J.-C.
- Parola P.
- et al.
- Fang L.
- Karakiulakis G.
- Roth M.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
| Medication | Dosing |
|---|---|
| Remdesivir | 200 mg for 1 day, then 100 mg IV every day for 9 days |
| Lopinavir/Ritonavir | 400–100 mg PO BID for 14 days |
| Chloroquine | 500 mg PO BID for 10 days |
| Hydroxychloroquine | 400 mg PO BID for 1 day, then 200 mg PO BID for 4 days |
| Tocilizumab | 8 mg/kg in 100 mL of 0.9% NS IV over 60 min |
| Favipiravir | 1600 mg PO BID for one day, then 600 mg PO BID for 6 days |
2.8 Disposition
World Health Organization. Home care for patients with suspected novel coronavirus (nCoV) infection presenting with mild symptoms and management of contacts. https://www.who.int/publications-detail/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts. Published February 4, 2020. Accessed February 24, 2020.
CDC. Coronavirus Disease 2019 (COVID-19): interim US guidance for risk assessment and public health management of persons with potential Coronavirus Disease 2019 (COVID-19) exposures: geographic risk and contacts of laboratory-confirmed cases. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/php/risk-assessment.html. Published March 7, 2020. Accessed March 20, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) flowchart for healthcare professionals. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/identify-assess-flowchart.html. Published February 11, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) flowchart for healthcare professionals. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/identify-assess-flowchart.html. Published February 11, 2020. Accessed March 19, 2020.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
3. Conclusion
Meetings
Grants/financial support
Author contributions
Declaration of competing interest
Acknowledgements
References
World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov). Published January 30, 2020. Accessed February 18, 2020.
World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Published March 11, 2020. Accessed March 20, 2020.
World Health Organization. WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Published February 11, 2020. Accessed March 08, 2020.
World Health Organization. Q&A on coronaviruses. https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. Published February 11, 2020. Accessed March 08, 2020.
New images of Novel Coronavirus SARS-CoV-2 now available|NIH: National Institute of Allergy and Infectious Diseases. https://www.niaid.nih.gov/news-events/novel-coronavirus-sarscov2-images. Accessed March 18, 2020.
CDC. 2019 ovel Coronavirus (2019-nCoV) frequently asked questions and answers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/faq.html. Published February 11, 2020. Accessed March 18, 2020.
Naming the coronavirus disease (COVID-2019) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. Accessed March 12, 2020.
CDC. HAN Archive - 00426|Health Alert Network (HAN). https://emergency.cdc.gov/han/han00426.asp. Published February 11, 2020. Accessed March 19, 2020.
- CIDRAP.http://www.cidrap.umn.edu/news-perspective/2019/12/news-scan-dec-31-2019Date: 2019Date accessed: March 18, 2020
Taylor DB. A timeline of the coronavirus. The New York Times. https://www.nytimes.com/2020/02/13/world/coronavirus-timeline.html. Published February 13, 2020. Accessed March 18, 2020.
- CHP closely monitors cluster of pneumonia cases on Mainland.https://www.info.gov.hk/gia/general/201912/31/P2019123100667.htmDate accessed: March 18, 2020
Salcedo A, Cherelus G. Coronavirus travel restrictions, across the globe. The New York Times. https://www.nytimes.com/article/coronavirus-travel-restrictions.html. Published March 20, 2020. Accessed March 20, 2020.
- 780 million people in China face travel restrictions over coronavirus outbreak. CNN.https://www.cnn.com/2020/02/16/asia/coronavirus-covid-19-death-toll-update-intl-hnk/index.htmlDate accessed: March 18, 2020
CDC. 2019 Novel Coronavirus (2019-nCoV) situation summary. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/summary.html. Published February 11, 2020. Accessed March 18, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) prevention & treatment. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/prevention-treatment.html. Published February 11, 2020. Accessed March 19, 2020.
- The impact of a case of Ebola virus disease on Emergency Department visits in Metropolitan Dallas-Fort Worth, TX, July, 2013–July, 2015: An interrupted time series analysis.PLoS Curr. 2018; : 10https://doi.org/10.1371/currents.outbreaks.e62bdea371ef5454d56f71fe217aead0
- Ebola virus disease: preparedness and infection control lessons learned from two biocontainment units.Curr Opin Infect Dis. 2015; 28: 343-348https://doi.org/10.1097/QCO.0000000000000176
Identify, Isolate, Inform: Emergency Department Evaluation and Management for Patients Under Investigation (PUIs) for Ebola Virus Disease (EVD)|Emergency Services|Clinicians|Ebola (Ebola Virus Disease)|CDC. https://www.cdc.gov/vhf/ebola/clinicians/emergency-services/emergency-departments.html. Published August 30, 2019. Accessed February 18, 2020.
Coronavirus|Human Coronavirus Types|CDC. https://www.cdc.gov/coronavirus/types.html. Published February 16, 2020. Accessed March 12, 2020.
- Early transmission dynamics in Wuhan, China, of Novel Coronavirus–infected pneumonia.N Engl J Med. 2020; 0 (null)https://doi.org/10.1056/NEJMoa2001316
CDC. Coronavirus Disease 2019 (COVID-19): animals and Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/prepare/animals.html. Published March 16, 2020. Accessed March 20, 2020.
- Hirsch M. Bloom A. Coronavirus disease 2019 (COVID-19). Date, February 2020
CDC. 2019 Novel Coronavirus (2019-nCoV) transmission. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html. Published February 11, 2020. Accessed March 12, 2020.
- Director-General's remarks at the media briefing on COVID-2019 outbreak on 17 February.https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-covid-2019-outbreak-on-17-february-2020Date: 2020Date accessed: March 17, 2020
World Health Organization. Coronavirus disease 2019 (COVID-19) situation report – 60. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200320-sitrep-60-covid-19.pdf?sfvrsn=8894045a_2. Published March 20, 2020. Accessed March 20, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) cases in the U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-in-us.html. Published March 20, 2020. Accessed March 20, 2020.
Coronavirus Disease 2019 Transcript for CDC Media Telebriefing. Centers for Disease Control and Prevention. https://wwwdev.cdc.gov/media/releases/2020/s0215-Diamond-Princess-Repatriation.html. Published February 18, 2020. Accessed March 19, 2020.
- SARS-CoV-2 viral load in upper eespiratory specimens of infected patients.N Engl J Med. 2020; 0 (null)https://doi.org/10.1056/NEJMc2001737
- Presumed asymptomatic carrier transmission of COVID-19.JAMA. February 2020; https://doi.org/10.1001/jama.2020.2565
- Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients.Can J Anesth Can Anesth. February 2020; https://doi.org/10.1007/s12630-020-01591-x
- COVID-19—new insights on a rapidly changing epidemic.JAMA, 2020https://doi.org/10.1001/jama.2020.3072 (February)
- Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020.Eurosurveillance. 2020; 25: 2000058https://doi.org/10.2807/1560-7917.ES.2020.25.4.2000058
Rettner R. How does the new coronavirus compare with the flu? livescience.com. https://www.livescience.com/new-coronavirus-compare-with-flu.html. Published March 19, 2020. Accessed March 11, 2020.
- The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020.China CDC Wkly. 2020; 2: 1-10
- Feb 17 LS|NE|CN|, 2020. More outbreak details emerge as COVID-19 cases top 70,000. CIDRAP.(Accessed March 19, 2020)
- Facts vs. fears: five things to help weigh your Coronavirus risk. Kais Health News.https://khn.org/news/facts-vs-fears-five-things-to-help-weigh-your-coronavirus-risk/Date: February 2020Date accessed: March 19, 2020
- Report 2: estimating the potential total number of novel Coronavirus cases in Wuhan City.China. 2020; 6
- Report 4: severity of 2019-novel coronavirus (nCoV).(Available at)
- Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China.The Lancet. 2020; 395: 497-506https://doi.org/10.1016/S0140-6736(20)30183-5
- Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study.The Lancet. 2020; 395: 507-513https://doi.org/10.1016/S0140-6736(20)30211-7
Parker E, The Vaccine Centre, London School of Hygiene & Tropical Medicine. Covid 2019 tracker. https://vac-lshtm.shinyapps.io/ncov_tracker/. Published 2020. Accessed March 21, 2020.
- Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017.Lancet Respir Med. 2019; 7: 69-89https://doi.org/10.1016/S2213-2600(18)30496-X
CDC. 2019 Novel Coronavirus (2019-nCoV) symptoms. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html. Published February 11, 2020. Accessed March 19, 2020.
- Clinical diagnosis of 8274 samples with 2019-novel coronavirus in Wuhan.medRxiv. February 2020; 2020 (02.12.20022327)https://doi.org/10.1101/2020.02.12.20022327
Shah N. Higher co-infection rates in COVID19. Medium. https://medium.com/@nigam/higher-co-infection-rates-in-covid19-b24965088333. Published March 19, 2020. Accessed March 20, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) clinical care. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) pregnant women. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/pregnancy-faq.html. Published February 11, 2020. Accessed March 19, 2020.
- What are the risks of COVID-19 infection in pregnant women?.The Lancet. 2020; 0https://doi.org/10.1016/S0140-6736(20)30365-2
CDC - Health Care Workers, Infectious Agents - NIOSH workplace safety and health topic. https://www.cdc.gov/niosh/topics/healthcare/infectious.html. Published November 6, 2018. Accessed March 12, 2020.
- Intubation of SARS patients: infection and perspectives of healthcare workers.Can J Anaesth J Can Anesth. 2006; 53: 122-129https://doi.org/10.1007/bf03021815
- Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area.JAMA. 2003; 289: 2801-2809https://doi.org/10.1001/jama.289.21.JOC30885
- The occupational risk of influenza A (H1N1) infection among healthcare personnel during the 2009 pandemic: a systematic review and meta-analysis of observational studies.PLoS ONE. 2016; 11https://doi.org/10.1371/journal.pone.0162061
American College of Emergency Physicians. Two emergency physicians in critical condition. ACEP. https://www.acep.org/corona/covid-19-articles/a-statement-from-acep-president-william-jaquis-md-facep/. Published March 14, 2020. Accessed March 21, 2020.
2 ER doctors at rush Oak Park Hospital test positive for Coronavirus. NBC Chic. March 2020. https://www.nbcchicago.com/news/local/2-doctors-at-rush-oak-park-hospital-test-positive-for-coronavirus/2240158/. Accessed March 21, 2020.
Italy has a world-class health system. The coronavirus has pushed it to the breaking point. NBC News. https://www.nbcnews.com/health/health-news/italy-has-world-class-health-system-coronavirus-has-pushed-it-n1162786. Accessed March 20, 2020.
- Mar 16 MVB|NW|CN|, 2020. Doctors: COVID-19 pushing Italian ICUs toward collapse. CIDRAP.(Accessed March 20, 2020)
CDC. 2019 Novel Coronavirus (2019-nCoV) flowchart for healthcare professionals. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/identify-assess-flowchart.html. Published February 11, 2020. Accessed March 19, 2020.
- A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster.The Lancet. 2020; 395: 514-523https://doi.org/10.1016/S0140-6736(20)30154-9
- Clinical characteristics of Coronavirus Disease 2019 in China.N Engl J Med. 2020; 0 (null)https://doi.org/10.1056/NEJMoa2002032
- Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China.JAMA. 2020; 323: 1061-1069https://doi.org/10.1001/jama.2020.1585
- Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study.(Published March 5. Available at)https://journals.lww.com/ajg/Documents/COVID_Digestive_Symptoms_AJG_Preproof.pdfDate: 2020Date accessed: March 21, 2020
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published January 28, 2020. Accessed March 19, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Infection Prevention and Control Recommendations. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html. Published February 11, 2020. Accessed March 20, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) healthcare infection prevention and control FAQs for COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-prevention-control-faq.html. Published February 11, 2020. Accessed March 19, 2020.
- COVID-19: what is next for public health?.The Lancet. 2020; 395: 542-545https://doi.org/10.1016/S0140-6736(20)30374-3
CDC. Coronavirus Disease 2019 (COVID-19): evaluating and testing persons for Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published February 11, 2020. Accessed March 21, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) evaluating and reporting PUI. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Published February 11, 2020. Accessed March 14, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) infection control. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published February 11, 2020. Accessed March 19, 2020.
- Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19).101. 2020
Public Health Agency of Canada. Infection prevention and control for coronavirus disease (COVID-19): interim guidance for acute healthcare settings. aem. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/interim-guidance-acute-healthcare-settings.html#a4.18. Published February 24, 2020. Accessed February 27, 2020.
Anesthesia Patient Safety Foundation. Perioperative considerations for the 2019 Novel Coronavirus (COVID-19). Anesthesia Patient Safety Foundation. https://www.apsf.org/news-updates/perioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published February 12, 2020. Accessed February 24, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) Testing. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. 2019 Novel Coronavirus (2019-nCoV) information for laboratories COVID-19 requests for diagnostic panels and virus. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/lab/tool-virus-requests.html. Published February 11, 2020. Accessed March 19, 2020.
CDC. Coronavirus Disease 2019 (COVID-19) interim guidelines for collecting, handling, and testing clinical specimens from persons under investigation (PUIs) for Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Published February 11, 2020. Accessed March 20, 2020.
- Detection of SARS-CoV-2 in different types of clinical specimens.JAMA. March 2020; https://doi.org/10.1001/jama.2020.3786
- Correlation of chest CT and RT-PCR testing in Coronavirus Disease 2019 (COVID-19) in China: a report of 1014 cases.Radiology. 2020 Feb; 26 ([Epub ahead of print]): 200642https://doi.org/10.1148/radiol.2020200642
Januzzi J. Troponin and BNP use in COVID-19. Latest in cardiology. http%3a%2f%2fwww.acc.org%2flatest-in-cardiology%2farticles%2f2020%2f03%2f18%2f15%2f25%2ftroponin-and-bnp-use-in-covid19. Published March 18, 2020. Accessed March 20, 2020.
- Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic.Intensive Care Med. March 2020;
World Health Organization. DRAFT landscape of COVID-19 candidate vaccines. World Health Organization. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf?ua=1. Published March 20, 2020. Accessed March 21, 2020.
- An interview with Andrea Duca, MD. EBMedicine.(Published March 19. Available at)https://www.ebmedicine.net/topics/infectious-disease/COVID-19/podcastDate: 2020Date accessed: March 22, 2020
- The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus.J Biol Chem. February 2020; (jbc.AC120.013056)https://doi.org/10.1074/jbc.AC120.013056
- Compounds with therapeutic potential against novel respiratory 2019 coronavirus.Antimicrob Agents Chemother. March 2020; (AAC.00399-20, aac;AAC.00399-20v1)https://doi.org/10.1128/AAC.00399-20
- Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro.Cell Res. 2020; 30: 269-271https://doi.org/10.1038/s41422-020-0282-0
- Drug treatment options for the 2019-new coronavirus (2019-nCoV).Biosci Trends. 2020; 14: 69-71https://doi.org/10.5582/bst.2020.01020
- Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies.Biosci Trends. 2020 Mar 16; 14: 72-73
- A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19.N Engl J Med. March 2020; https://doi.org/10.1056/NEJMoa2001282
- Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial.Int J Antimicrob Agents. March 2020; 105949https://doi.org/10.1016/j.ijantimicag.2020.105949
- Tocilizumab vs CRRT in management of cytokine release syndrome (CRS) in COVID-19 (TACOS).
- Favipiravir combined with tocilizumab in the treatment of Corona Virus Disease.
- Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target.Intensive Care Med. March 2020; : 1-5https://doi.org/10.1007/s00134-020-05985-9
- Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?.Lancet Respir Med. 2020; 0https://doi.org/10.1016/S2213-2600(20)30116-8
World Health Organization. Home care for patients with suspected novel coronavirus (nCoV) infection presenting with mild symptoms and management of contacts. https://www.who.int/publications-detail/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts. Published February 4, 2020. Accessed February 24, 2020.
CDC. Coronavirus Disease 2019 (COVID-19): interim US guidance for risk assessment and public health management of persons with potential Coronavirus Disease 2019 (COVID-19) exposures: geographic risk and contacts of laboratory-confirmed cases. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/php/risk-assessment.html. Published March 7, 2020. Accessed March 20, 2020.


