ALARMED: Adverse events in Low-risk patients with chest pain Receiving continuous electrocardiographic Monitoring in the Emergency Department. A pilot study
Brief Reports
ALARMED: Adverse events in Low-risk patients with chest pain Receiving continuous electrocardiographic monitoring in the Emergency Department. A pilot study
Clare Atzema MDa,*, Michael J. Schull MD, MSc, FRCPCa,
Bjug Borgundvaag PhD, MD, CCFP(EM)b, Graham R.D. Slaughter PhDc,
Cheong K. Lee BScc
aDivision of Emergency Medicine, Sunnybrook and Women’s College Health Sciences Centre, Toronto, ON, Canada M4N 3M5
bSchwartz/Reisman Emergency Centre, Mount Sinai Hospital, Toronto, ON, Canada M5G 1X5
cLondon Health Sciences Centre, London, ON, Canada N6A 5W9
Accepted 2 May 2005
Abstract
Objectives: Current guidelines suggest that most patients who present to an emergency department (ED) with chest pain should be placed on a continuous electrocardiographic monitoring (CEM) device. We evaluated the utility of CEM in ED patients with chest pain.
Methods: We enrolled stable patients who presented to a single ED with chest pain suspected to be
ischemic in origin and who were placed on CEM. Patients were classified according to risk of poor outcome using 3 published stratification tools. Trained observers prospectively recorded number of monitored hours, alarms, changes in management, and monitor-detected adverse events (AEs). The primary outcome measure was the rate of AEs detected by CEM. Secondary outcome measures were the rate of alarms that resulted in a Change in management and number of false alarms.
Results: We enrolled 72 patients, 56% of whom were categorized as very low-risk by Goldman risk
criteria. During 371 monitored hours, we recorded 1762 alarms or 4.7 alarms per monitored hour. There were 11 AEs (0.68%; 95% CI, 0.35%-1.2%), 3 of which resulted in a change in management (0.2%; 95% CI, 0.04%-0.5%). Seven AEs were bradydysrhythmias with a heart rate of 45 or higher; the eighth patient had no change in symptoms and was given atropine for a heart rate of 32. The other 3 AEs were an untreated supraventricular tachycardia, a brief sinus pause that triggered a rate change in Intravenous nitroglycerin by the patient’s nurse, and a run of premature ventricular contractions after which heparin was administered. None of the 3 patients with a change in management was categorized as the lowest-risk. Conclusions: Routine CEM in low-risk ED patients with chest pain results in an excessive number of alarms, most of which require no change in management. In these patients, the benefit of CEM may be limited, and given that 99.4% of alarms were false, current CEM technology needs to be improved.
D 2006
Presented at the Canadian Association of Emergency Physicians (CAEP) Annual Scientific Meeting in Montreal, April 2004.
T Corresponding author. Tel.: +1 416 390 0739 (Pager).
E-mail address: [email protected] (C. Atzema).
0735-6757/$ - see front matter D 2006 doi:10.1016/j.ajem.2005.05.015
Introduction
Currently in the United States and Canada, most patients who present to an ED with chest pain are placed on a continuous electrocardiographic monitor (ie, a telemetry monitor). Because of an increased risk of a fatal arrhythmia in patients experiencing an acute myocardial infarction [1,2] and the effectiveness of Early defibrillation for dysrhythmia termination [3], both the American Heart Association and the Canadian Association of Emergency Physicians stipulate that patients with chest pain that could be due to ischemia be placed on Continuous ECG monitoring (CEM) [4,5]. These guidelines are based on expert opinion, as there are no published clinical trials on monitoring for brule-out myocardial infarctionQ patients in the ED [4,5]. Several studies have questioned the use of CEM in admitted patients with chest pain [6], citing negligible rates of adverse events (AEs) in bvery low-riskQ patients in particular [7-9]. ED patients with chest pain are significantly different from their admitted counterparts because (1) if they are suffering an acute coronary syndrome (ACS), they are at a significantly higher risk of having an AE early in the course of their disease when they are in the ED [10], and (2) because the majority (more than 80%) of these patients are not actually experiencing an ACS [11], this dilutes the number of AEs in the ED chest pain patient population. Approximately 70% of ED patients with chest pain have no ACS nor an AE [12]. Although studies of
continuous ST-segment monitoring have been conducted in ED patients [13], currently, most EDs do not use this technology [4], and no study has evaluated the use of standard CEM in ED patients with chest pain.
ED crowding is worsening [14,15], and because patients with chest pain are subject to these forces [16], the availability of monitored beds for these patients has decreased. If all patients with cardiac-related chest pain, including low-risk patients, use CEM, sicker patients who will yield a greater benefit may be denied the technology because of a limited number of monitors [7,16]. In addition, ED crowding is exacerbated by time spent in the ED awaiting a monitored ward bed [17], and monitoring carries a Financial burden through increased nursing time and equipment costs [14]. These monitors could be allocated more efficiently if emergency physicians (EPs) could reliably identify a subgroup of patients with chest pain with a Very low risk of having a monitor-signaled AE. We suspect that in the current practice environment, EPs are frequently faced with fully occupied ED monitors and are using their clinical judgment to decide which patients to remove from monitoring when a higher acuity patient arrives. In this situation in particular, evidence-based criteria for monitor removal would be very useful.
We conducted this pilot study to test the feasibility of a large, prospective, observational study on the utility of CEM in ED patients with chest pain. In this descriptive study, we determined the rate of AEs detected by CEM, the rate of
Table 1 Definition of alarm types (standard machine settings), AEs and changes in management
Red arrhythmia alarm Yellow arrhythmia alarm Bed alarm
Alarm HR b45 or N150 Pair PVCs Monitor not picking up patient signals (ie, reads apnea when saturation probe is not on patient’s finger)
Asystole Multiform PVC Ventricular tachycardia HR b50 bpm Ventricular fibrillation HR N130 bpm
Irregular HR R on T PVC PVCs N10
ventricular bigeminy Pacer not capturing Pause
Arrhythmia adverse event
Asystole or pulseless electrical activity
Ventricular fibrillation or ventricular tachycardia Bradydysrhythmia (HR b50), supraventricular tachydysrhythmias (HR N140) or atrial fibrillation/flutter (HR N100)
New second or third degree heart block Fluid bolus or change in intravenous rate Use of pressors
Other drug therapy
Electrical therapy (defibrillation)
oxygen flow rate change or use of BiPap or intubation Need for immediate medical doctor reassessment of patient Decision to refer to specialty service
R on T indicates a highgrade (high risk for ventricular arrhythmia; PVC, premature ventricular contraction).
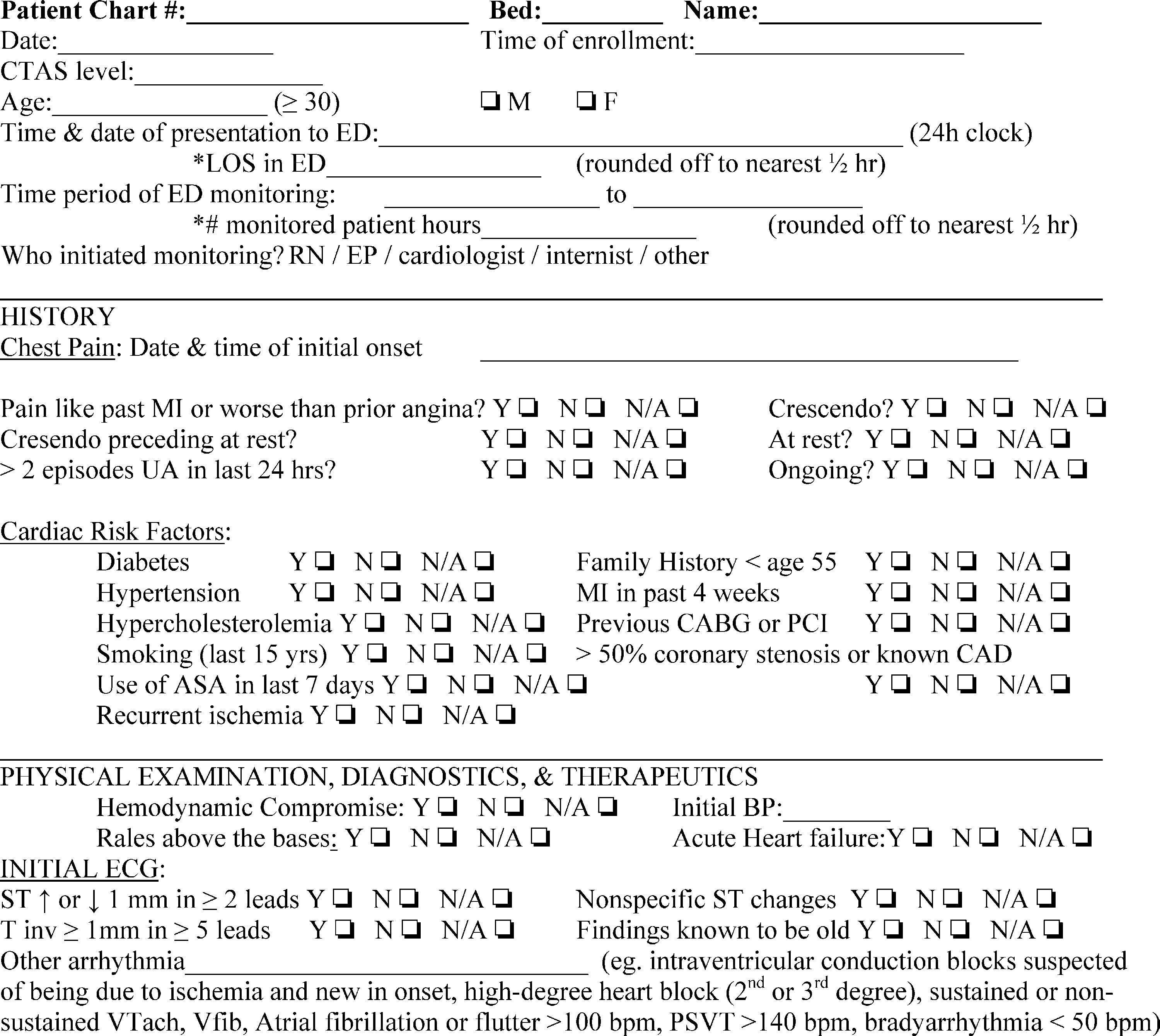
Fig. 1 Standardized data collection instrument.
change in management that occurred secondary to alarms, and the rate of false alarms that occurred during CEM.
Methods
Study design
We conducted a prospective, observational cohort study of patients with chest pain who were placed on CEM in the ED from June to August 2003. Care was directed by the managing physician and was not influenced by the study. Subjects were enrolled consecutively from 11:00 until 23:00, Monday to Friday. The protocols were reviewed and approved by the hospital research ethics board.
Setting
The study took place at Mount Sinai Hospital in Toronto, Ontario, a tertiary care teaching hospital ED with an annual census of 36 000. The hospital has a 24 - hour on-call cardiology service and a cardiac catheterization laboratory. The ED is equipped with the Agilent Model # M3150A monitoring system (Agilent Technologies Canada Inc, Mis- sissanga, ON, Canada), which does not provide ST-segment analysis or scheduled serial ECGs. Fourteen beds are routinely monitored using bedside monitors, which also display the rhythms on central monitors at 2 nursing stations.
Patients
We enrolled patients 30 years or older who presented to the study center ED with atraumatic chest pain that the managing EP (either staff EP or emergency medicine resident) felt could be cardiac in origin and who were placed on CEM (which was decided by either the triage nurse or the managing EP). We excluded patients who had either ST deviation of 1 mm or higher in 2 contiguous leads or T-wave inversion in 5 leads or more on their initial ECG [18], which the managing physician felt represented cardiac ischemia. We also excluded hemodynamically unstable patients, patients with a predefined arrhythmia on initial ECG (Table 1, arrhythmia adverse event), or patients who were transferred from another hospital.
A daily review of ED triage logs for all patients with the chief complaint of chest pain and a Canadian Triage and Acu- ity Scale score of I, II, or III (the highest Acuity scores) was conducted to identify the number of missed eligible patients.
Data collection
Data were collected by 2 trained investigators (GRDS and CKL). After initial Patient evaluation by the managing EP, this physician was asked to fill out a standardized data collection instrument (Fig. 1). Patients were categorized according to risk of AEs using the Goldman [10], Canadian
documented on the data collection sheet at a maximum of every 2 hours. Types of alarms were red (serious arrhyth- mias), yellow (less serious arrhythmias), or bed alarms (minor alarms). The investigators presented the list of alarms documented over the previous 2 hours to the patient’s nurse and the nurse was interrogated for any predefined AEs or changes in management that had occurred because of the alarms (Table 1). Any break in monitoring was noted (eg, to leave the department for an x-ray), and if greater than 15 minutes, the time was rounded up to 30 minutes and subtracted from the total monitored hours. Once the patient was admitted and left the ED and/or monitoring was discontinued, we collected data on the use of Cardiac interventions, thrombolysis, cardiac enzyme levels, and place of discharge from the ED. Six months later, the principal investigator accessed inpatient hospital charts and computerized lists of outpatient visits to collect the presence of any follow-up visits to the hospital cardiology clinic and any cardiac interventions (including exercise Stress testing, any perfusion imaging, echocardiogram, or coronary artery bypass graft/percutaneous coronary inter- vention/angiogram).
RF, risk factors; CHRC, Canadian Heart Research Centre.
a Troponin T, 1.82; second set, 1.71 (patient had been discharged the day before after acute myocardial infarction and RCA stent placement the week before).
b Troponin T as above for first patient; second patient: first troponin T 0.05; second set, 0.21.
c This patient is also included in the bpositiveQ cardiac catheteri- zation group.
d One stress echocardiogram was performed 3 months after presentation in the ED.
3 (2 catheterized) 5d (1 catheterized)
4
1
45 (63%)
5 (7%)
21 (30%)
1c
Coronary artery
bypass graft
Stress echocardiogram Echocardiogram exercise stress test Place of Discharge home
Hospital ward without CEM Hospital ward with CEM
Negative
4
Cardiac catheterization 3
(angiogram F stent)
2b/41 Positive
1a/71
Troponin T levels
Initial set troponin T (positive is 0.10ug/L) Second set troponin T Interventions
High: 2 (3%)
Moderate:
15 (21%)
Low: 14 (20%)
Very low:
40 (56%)
0 or 1 RF: 42 (59%)
2 RF: 10 (14%)
3 RF: 13 (18%)
4 RF: 3 (4%)
5 RF: 2 (3%)
6 RF: 1 (1%)
7 RF: 0
High: 10 (14%)
Intermediate: 21 (30%)
Low: 40 (56%)
CHRC criteria [19]
TIMI criteria [18]
Table 2 Characteristics of 72 patients with chest pain,
interventions and place of discharge in 71 patients
Patient characteristic Number of
patients (%)
Demographics
Age 57 (range, 30-90)
Sex 40 male (56%)
Published risk stratification tools Goldman criteria [10]
Outcome measures
The primary outcome measure was the rate of AEs detected by CEM during the period of monitoring in the ED, which was measured per total alarms as well as per monitored hour. Adverse events were categorized as bvital signQ and barrhythmiaQ events. Vital sign AEs were defined as an episode of an abnormality in pulse oximetry, heart rate (HR), or blood pressure sufficient to warrant a change in management. Arrhythmia events are defined in Table 1. Secondary outcome measures were the frequency with which monitor alarms resulted in a change in management per all alarms as well as per monitored hour and the rate of false alarms during the CEM period.
Data analysis
Statistical analysis was performed using simple propor- tions with 95% confidence intervals (CIs), using Excel software (Microsoft, Redmond, WA).
Heart Research Centre (CHRC) [18], and Thrombolysis in Myocardial Infarction [19] criteria.
The centralized CEM computer system records the time, type, and reason for all alarms; these logs were reviewed and
Results
We identified 86 patients who were eligible for study enrolment. Thirteen patients refused consent or were unable to consent because of a language barrier. Another patient left against medical advice before consent. Thus, we en- rolled 72 patients; patient characteristics are presented in Table 2. One enrolled patient had insufficient data collected to characterize his risk with the stratification tools. Using triage logs, we identified 1 eligible patient who was missed for enrolment.
|
Moderate |
2 |
Intermediate |
Bradycardia 45-50 |
|
|
72F |
Moderate |
3 |
High |
Bradycardiab 45-50 |
|
54F |
Very low |
1 |
Intermediate |
Bradycardia 45-50 |
|
54F |
Moderate |
1 |
High |
Bradycardia 45-50 |
|
50M |
Low |
3 |
Intermediate |
Sinus pause |
|
48F |
Moderate |
0 |
Intermediate |
SVT 150 |
|
56M |
Low |
4 |
Intermediate |
Bradycardia 45-50 |
|
69M |
Moderate |
4 |
High |
PVCs N10 |
On initial ECG, 19 (26%) patients had nonspecific changes. Nine (13%) had either ST deviation of 1 mm or higher in 2 contiguous leads or T wave inversion of 1 mm or higher in 5 or more leads; however, because the managing EPs did not think that these findings represented ischemia, these patients were included in the study.
Table 3 Eleven patients with adverse events
Patient Goldman risk
63F Very low
72M Low
78M Moderate
TIMI risk
1
5
3a
CHRC risk
Intermediate Intermediate Intermediate
Adverse event
Bradycardia 45-50
Bradycardia 40-45
Bradycardia 32
M, male; F, female; SVT, supraventricular tachycardia; PVC, premature ventricular contraction.
a Patient 05 actually had 4 TIMI risk factors, with an angiogram showing coronary stenosis between 60% to 100% in main vessels. The information was not available during the ED stay.
b Patient 08 was in atrial fibrillation, and had chronic digoxin toxicity that was evident on initial ECG; HR b50 was transient over several beats at most as the rhythm was irregular.
The 71 patients spent an average of 5 hours (SD, 4 hours) on CEM and used 371 monitored hours; during which time, we recorded 1762 alarms (1019 arrhythmia alarms and 743 bed alarms). This produced a rate of 4.7 alarms per monitored hour. There were 11 AEs identified by alarms for an AE detection rate of 0.62% (95% CI, 0.31%-1.1%). The rate of AEs per monitored hour was 0.030 (95% CI, 0.015-0.052), or 1 AE every 33 monitored hours. There was
1 vital sign event, whereas the other 10 AEs were arrhythmia events. None were hemodynamically significant. Three AEs were associated with a change in management for a rate of 0.2% (95% CI, 0.04%-0.5%) per alarm, and 1 change in management for every 124 hours of CEM. None of the 3 patients with a change in management were categorized as the lowest risk by the stratification tools.
The one patient who had a vital sign AE entered the study with an HR in the low fifties, had chronic atrial fibrillation, and was taking metoprolol twice daily. His rate slowly decreased during his 8 hours of CEM, and he was given atropine when his HR was 32. His symptoms did not change during that time. Of the arrhythmia AEs, 7 were bradydysrhythmias: 7 patients had an HR of 45 to 50 beats per minute (bpm), none of whom required treatment for it. The other 3 arrhythmia events were a transient supraven- tricular tachycardia that required no therapy, a brief sinus pause that triggered a change in rate of intravenous nitroglycerin infusion and closer monitoring by the patient’s nurse, and a run of premature ventricular contractions (PVCs), after which heparin and clopidogrel were admin- istered. Risk scores for AE patients are listed in Table 3.
With 11 AEs per 1762 alarms, the false alarm rate was 99.4% (95% CI, 98.9%-99.7%). There were no deaths. Further investigations are listed in Table 2. Most patients were discharged home from the ED (63%).
Discussion
The utility of CEM among patients admitted to hospital for known or potential ischemic heart disease has been questioned because of the low rate of AEs identified by the technology [5-7,15]. Our study reached a similar conclusion among ED patients with chest pain. This small, prospective study found that only 0.62% of CEM alarms were sounded because of an AE, none of which were hemodynamically significant, and only 0.2% resulted in a change in management. This is despite a significant proportion of the group falling outside the lowest risk categories accord- ing to published risk stratification criteria. In addition, the AEs of 7 of the 11 patients with an AE might not be termed AEs at all, as an HR of 45 to 50 likely represents concordance with the recommended use of b-blockers in ACS patients.
However, it must be emphasized that the small sample size and/or power of our study prevents us from drawing firm conclusions about the rate of AEs detected by CEM, and the single study center limits the generalizability of our results. Our most interesting finding, however, was the extremely large number of alarms (1762) during the
371 monitored hours. This is the first study to record number of alarms during CEM in any setting, and we found that more than 99% of them were false. This is a very low signal-to-noise ratio and calls into question both the efficiency of CEM technology, as well as how we use it (ie, perhaps we should alter standard machine settings, find ways to improve lead connections, etc). Of even greater
importance is that false alarms may not only be annoying, but they may also have adverse effects of their own. False alarms are often silenced by the patient’s nurse, resulting in interruptions, distractions, and likely significant amounts of wasted time. The effect is likely to be worse in a crowded ED. In addition, their frequency likely decreases both nurse and physician sensitivity to alarms. Finally, when more than 99% of alarm signals are false, there is a real risk that some may be misinterpreted and acted upon in error, resulting in unnecessary investigations, referral, or treatment [4].
A final limitation of this study was the inclusion of some high-risk patients in our patient group. When the managing EP decided that certain ECG findings did not represent ischemia, these patients were included in the study, although they were still classified as high-risk by the stratification tools. In fact, 4 of the patients went on to have a positive cardiac catheterization study, suggesting that these patients were indeed high-risk. Our study protocol and exclusion criteria will need to be revised in future large-scale studies.
Conclusions
The use of CEM in patients with chest pain in the ED may be of limited value, both because of a low rate of monitor-signaled AEs and in the high number of false alarms. This resource appears to be very inefficient in its current role: given that 99.4% of alarms were false, current CEM technology needs to be improved. A large prospective study is warranted to further evaluate the utility of this resource in ED low-risk patients with chest pain.
Acknowledgment
The authors thank Michelle Loftus, research coordinator, for her valuable research assistance.
References
- Julian DG, Valantine PA, Miller GG. Disturbances of rate, rhythm and conduction in acute myocardial infarction. Am J Med 1964;37:915 - 27.
- Spann JF, Moellering RC, Haber E, et al. Arrhythmias in acute myocardial infarction. A study utilizing an electrocardiographic monitor for automatic detection and recording of arrhythmias. N Engl J Med 1964;271:427 - 31.
- Zoll PM, Linethal AJ, Gibson W, et al. Termination of ventricular fibrillation in man by externally applied countershock. N Engl J Med 1956;254:727 - 32.
- Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings. An American Heart Association Scientific Statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Circulation 2004;110:2721 - 46.
- Beveridge R, Clarke B, Janes L, et al. Implementation guidelines for the Canadian emergency department triage and acuity scale (CTAS). www.caep.ca [Accessed January 3, 2004].
- Estrada CA, Rosman HS, Prasad NK, et al. Role of Telemetry monitoring in the non-intensive care unit. Am J Cardiol 1995; 76:960 - 5.
- Durairaj L, Reilly B, Das K, et al. Emergency department admissions to inpatient cardiac telemetry beds: a prospective cohort study of risk stratification and outcomes. Am J Med 2001;110(1):7 - 11.
- Hollander JE, Valentine SM, McCuskey CF, et al. Are monitored telemetry beds necessary for patients with nontraumatic chest pain and normal or nonspecific electrocardiograms? Am J Cardiol 1997; 79(8):1110 - 1.
- Hollander JE, Sites FD, Pollack CV, et al. Lack of utility of telemetry monitoring for identification of cardiac death and life-threatening ventricular dysrhythmias in low-risk patients with chest pain. Ann Emerg Med 2004;43:71 - 6.
- Goldman L, Cook EF, Johnson PA, et al. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996;334(23):1498 - 504.
- Pope JH, Aufderheide TP, Ruthazer R, et al. missed diagnoses of Acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163 - 70.
- Christenson J, Innes G, McKnight D, et al. Safety and efficiency of emergency department assessment of chest discomfort. CMAJ 2004; 170(12):1803 - 7.
- Fesmire FM, Percy RF, Bardoner JB, et al. Usefulness of automated serial 12-lead ECG monitoring during the initial emergency depart- ment evaluation of patients with chest pain. Ann Emerg Med 1998; 31(1):3 - 11.
- Derlet RW, Richards JR, Kravitz RL. Frequent overcrowding in US emergency departments. Acad Emerg Med 2001;8(2):151 - 5.
- Schull MJ, Szalai P, Schwartz B, et al. Emergency department overcrowding following systematic hospital restructuring: trends at twenty hospitals over ten years. Acad Emerg Med 2001;8(11):1037 - 43.
- Schull MJ, Morrison LJ, Vermeulen M, et al. Emergency department overcrowding and Ambulance transport delays for patients with chest pain. CMAJ 2003;168(3):277 - 8.
- Bayley MD, Sanford Schwartz J, et al. The financial burden of ED congestion and Hospital overcrowding for chest pain patients awaiting admission. Acad Emerg Med 2002;9(5):367.
- Fitchett D, Goodman S, Langer A. New advances in the management of acute coronary syndromes: 1. Matching treatment to risk. CMAJ 2001;164(9):1309 - 16.
- Antman EM, Cohen M, Bernink PJLM, et al. The TIMI risk score for unstable angina/non-ST elevation MI a method for prognostication and therapeutic decision making. JAMA 2000;284(7):835 - 42.
