A systematic review of nicardipine vs labetalol for the management of hypertensive crises
American Journal of Emergency Medicine (2012) 30, 981-993

Review
A systematic review of nicardipine vs labetalol for the management of Hypertensive crises?
W. Frank Peacock IV MD a,?, Daniel E. Hilleman PharmD b, Phillip D. Levy MD c,
Denise H. Rhoney PharmD d, Joseph Varon MD e
aDepartment of Emergency Medicine E19, The Cleveland Clinic, Cleveland, OH 44195, USA
bCreighton University Medical Center, Omaha, NE 68131, USA
cWayne State University Department of Emergency Medicine and Cardiovascular Research Institute, Detroit, MI, USA
dEugene Applebaum College of Pharmacy and Health Sciences, Wayne State University, Detroit, MI, USA
eThe University of Texas Health Science Center at Houston, Houston, TX, USA
Received 12 May 2011; revised 27 May 2011; accepted 30 June 2011
Abstract Hypertensive emergencies are acute elevations in Blood pressure that occur in the presence of progressive end-organ damage. Hypertensive urgencies, defined as elevated BP without acute end-organ damage, can often be treated with oral agents, whereas hypertensive emergencies are best treated with intravenous titratable agents. However, a lack of head-to-head studies has made it difficult to establish which intravenous drug is most effective in treating hypertensive crises. This systematic review presents a synthesis of published studies that compare the antihypertensive agents nicardipine and labetalol in patients experiencing acute hypertensive crises. A MEDLINE search was conducted using the term “labetalol AND nicardipine AND hypertension.” Conference abstracts were searched manually. Ultimately, 10 studies were included, encompassing patients with hypertensive crises across an array of indications and practice environments (stroke, the emergency department, critical care, surgery, pediatrics, and pregnancy). The results of this systematic review show comparable efficacy and safety for nicardipine and labetalol, although nicardipine appears to provide more predictable and consistent BP control than labetalol.
(C) 2012
Introduction
Hypertension affects approximately 1 billion people worldwide, 1% to 2% of whom will experience a Hypertensive crisis during their lifetime [1-3]. The Seventh
? Editorial support was provided by Jane Bryant, PhD, of Anthemis Consulting Ltd and was funded by EKR Therapeutics, Bedminster, NJ. The authors were not compensated and retained full editorial control over the content of this paper.
* Corresponding author.
E-mail address: [email protected] (W.F. Peacock).
Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of high blood pressure defines a hypertensive crisis as an acute elevation of systolic blood pressure (SBP) above 180 mm Hg or diastolic blood pressure above 120 mm Hg [1]. Hypertensive crises are associated with high rates of mortality; a study of practice patterns and outcomes in severe acute hypertension across 25 US hospitals reported that 90-day mortality rates are 11% in patients admitted and treated in the emergency setting [4].
Hypertensive crises are often the result of poorly controlled hypertension due to either inadequate treatment or compliance issues. The acute elevations in blood pressure
0735-6757/$ - see front matter (C) 2012 doi:10.1016/j.ajem.2011.06.040
(BP) that are characteristic of hypertensive crises are thought to arise from a sudden and Rapid increase in systemic vascular resistance caused by, among other things, sympa- thetic nervous system activation and release of humoral vasoconstrictors [5,6]. This acute rise in BP leads to mechanical stress and endothelial injury and can trigger a coagulation cascade culminating in arteriole necrosis and downstream ischemia [7,8]. Such processes cause inflam- mation and the release of additional vasoactive mediators, thus creating a positive feedback loop that continues to increase vasoconstriction, potentially leading to pervasive ischemia (especially in watershed regions) and end-organ hypoperfusion [5,6]. Furthermore, a subset of patients will develop intravascular hypovolemia as a result of the renal
response to Severe hypertension (ie, increased urine production and sodium excretion) and reduced fluid intake as the episode progresses. Not only can hypovolemia contribute to end-organ damage, it also creates a clinical situation where treatment to lower BP gives very unpredict- able responses, including inadvertent hypotension potentially leading to iatrogenic end-organ damage [5,9].
Hypertensive crises have been subdivided into hyperten- sive urgencies and emergencies [9-12]. Between 60% and 80% of hypertensive crises are hypertensive urgencies, and 20% to 40% are hypertensive emergencies [13,14]. Hyper- tensive urgencies are characterized by extreme BP elevations in the absence of progressive target organ dysfunction. Selected hypertensive urgencies require minimal treatment.
Table 1 Characteristics of nicardipine and labetalol
Characteristic Nicardipine Labetalol
Class of agent Second-generation dihydropyridine-derivative
FDA-approved dose 5 mg/h; increase at 2.5-mg/h increments every 5 min to a maximum of 15 mg/h
Available formulations IV (including premixed, ready-to-use formulation) and Oral formulations
Key features High vascular selectivity with strong cerebral and
coronary vasodilatory activity [20]
Combined selective ?1-adrenergic receptor blocker and nonselective ?-adrenergic blocker with an ?:? blocking ratio of 1:7 [19]
20 mg initial bolus; 20-80 mg repeat boluses or start infusion at 1-2 mg/min, with maximum dose of 300 mg in 24 h
IV bolus or continuous infusion (stored in a vial for preparation in a larger volume of solution)
Reduces systemic vascular resistance while cerebral, renal, and Coronary blood flows are maintained [24-27]
Crosses the blood-brain barrier The ?1-blocking component of labetalol minimized
Increases both stroke volume and coronary blood flow to increase myocardial oxygen balance [21,22] Rapidly accumulates in ischemic tissue causing localized vasodilation [23]
Does not cause venodilation
Nicardipine dosage is independent of patient weight. Not associated with coronary steal
the reductions in cardiac output seen with agents that have only ?-blocking activity [28].
Negligibly lipid soluble and, therefore, very little labetalol crosses the placenta, making it useful in the treatment of gestational hypertension [26].
Onset of action 5-15 min [29] 2-5 min [30]
Duration of hypotensive effects
Terminal half-life with IV administration
15-30 min, may exceed 4h [3] ~2-4 h [30]
Metabolism Nicardipine is rapidly and extensively metabolized by
the liver. Nicardipine does not induce or inhibit its own metabolism and does not induce or inhibit hepatic microsomal enzymes [29].
Metabolized by the liver to form an inactive glucuronide conjugate [30]
Contraindications and warnings
Should not be used in patients with advanced aortic stenosis. Patients with angina, heart failure, impaired Hepatic function, portal hypertension, or Renal impairment should be monitored closely when treated with nicardipine.
Caution is necessary in patients with heart failure. Labetalol is contraindicated in patients with severe Sinus bradycardia, asthma, chronic obstructive pulmonary disease, and greater than first-degree heart block.
Pregnancy category Category C: should only be used during pregnancy if
the potential benefits justify the possible risks to the fetus
Inclusion in guidelines Recommended by AHA/ASA for the treatment of
ischemic stroke when SBP N220 mm Hg or DBP
N120 mm Hg [31,32]
FDA indicates Food and Drug Administration.
Category C: should only be used during pregnancy if the potential benefits justify the possible risks to the fetus
Recommended by AHA/ASA for the treatment of ischemic stroke when SBP N220 mm Hg or DBP N120 mm Hg [31,32]
For example, in the setting of isolated systolic hypertension, the risk of acutely lowering BP may outweigh its benefits, and these cases may be best treated as patients with chronic hypertension, namely, with outpatient titration of Oral medication over several days [15]. Excessive BP lowering in patients with hypertensive urgency can result in adverse events such as Neurologic dysfunction and cerebral ischemia/ infarction [9-12].
Hypertensive emergencies are defined by severe eleva- tions in BP accompanied by acute end-organ dysfunction. The presence of end-organ damage in patients experiencing a Hypertensive emergency means that rapid, controlled re- ductions in BP are essential, although immediate attainment of “normal” BP levels in patients with such severe hypertension may not be desirable due to the potential for end-organ hypoperfusion. Hypertensive emergencies occur more frequently in African Americans and in the elderly and are twice as common in men as in women [16,17]. In addition, patients who experience hypertensive emergency tend to have higher DBP than patients who experience hypertensive urgency.
Hypertensive emergencies are best treated with intrave- nous (IV) titratable agents, so that controlled reductions in BP are achieved [9]. The recommendations of the Seventh Report of the Joint National Committee on Prevention,
Detection, Evaluation and Treatment of High Blood Pressure state that arterial BP should be reduced by no more than 10% to 25% during the first hour of treatment, although Volume depletion in these patients may warrant a more conservative approach. One strategy is to provide Volume expansion with IV saline while administering IV antihypertensives. The goal of this treatment approach is to attain an initial decline in DBP of 10% to 15% to address the immediate hypertensive concerns and to simultaneously prevent sudden falls in BP due to Severe dehydration. Once the patient’s BP has been stabilized and acute end-organ damage halted, IV antihyper- tensive medication can be down-titrated and replaced with oral agents.
A lack of comparative data in the acute care setting has left clinicians with little evidence-based guidance as to the optimal agent for BP control in patients with hypertensive crises. In fact, one study found that only 6% of patients who met the definition of severe hypertension had all the recommended tests [18]. This study demonstrated an incongruity between Guideline recommendations and routine practice, but as the guidelines were not evidence based, Karras and colleagues [18] questioned whether these findings represented subOptimal care or suboptimal guidelines. Furthermore, in a multicenter study of more than 1500 patients with acute severe hypertension, the
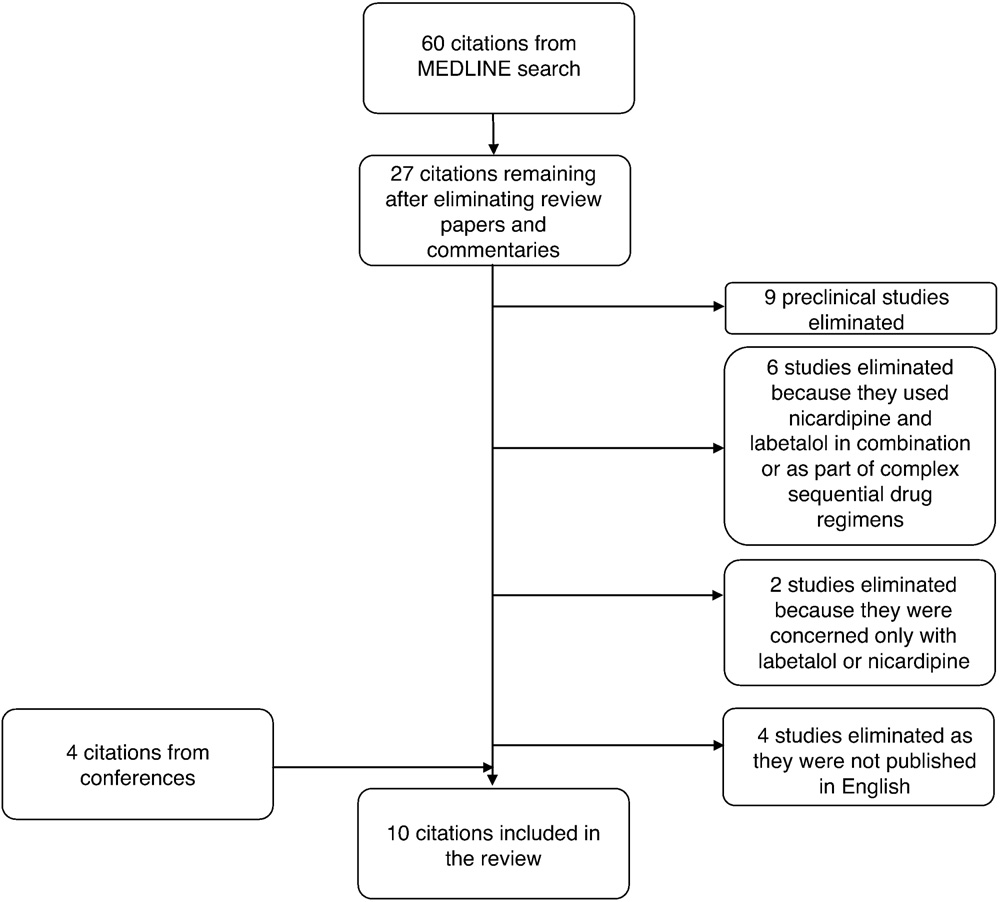
Fig. 1 Flow of studies through the systematic review.
Table 2 Summary of the included studies
Study (no. of nicardipine/labetalol patients)
Study design Results
Powers et al [34] (7/7)

- Population: patients with nontraumatic ICH with MAP 120-160 mm Hg
- Mean nicardipine MAP reduction +- SD: -23.4 +- 7.4 mm Hg (-15.6% +- 4.3%)
- Study duration: 6-22 h o Mean labetalol MAP reduction +- SD: -24.1 +- 8.4
- Nicardipine dose: 2-8 mg initial IV bolus followed by 2- to 15-mg/h infusion
- Labetalol dose: repeated 5-50 mg IV boluses up to a cumulative maximum dose of 200 mg
- Tx target: 15% MAP reduction
mm Hg (-17.8% +- 6.6%)
Liu-DeRyke et al o Population: patients with AIS, ICH, or SAH o Mean variability in MAP: nicardipine, 8.19 mm
[35] (26/64)
- Study duration: 24 h
- Mean nicardipine dose: 5 mg/h
- Mean labetalol total dose: 40 mg
- Tx target:
- SBP b180 mm Hg/DBP b105 mm Hg in patients with AIS eligible for thrombolytic therapy
- 10%-15% reduction from baseline MAP in patients with AIS not eligible for thrombolytic therapy
- 10%-15% MAP reduction in patients with ICH
- SBP b160 mm Hg in patients with aneurysmal SAH
Hg; labetalol, 10.78 mm Hg (P = .003)
- Patients with ICH who achieved target BP in 1 h: nicardipine, 33%; labetalol, 6% (P b .02)
- Mean no. of dosage adjustments: nicardipine, 2; labetalol, 4 (P b .001)
- Additional agents: nicardipine, 8%; labetalol, 33% (P = .013)
Martin-Schild et al o Population: patients with AIS and hypertension o Patients who reached Tx target in 3 h: aggressive
- (42 a/26 b)
- Study duration: 3 h
- Tx target: SBP/DBP b185/110 mm Hg
therapy, 48%; Standard therapy, 52%
-
- Hospital LOS: aggressive therapy, 4 d; standard therapy, 7 d (P = .01)
Liu-DeRyke et al

- Population: patients with AIS, ICH, or SAH
- Study duration: 24 h
- Tx target:
- SBP b180 mm Hg/DBP b105 mm Hg in patients with AIS eligible for thrombolytic therapy
- 10%-15% reduction from baseline MAP in patients with AIS not eligible for thrombolytic therapy
- 10%-15% MAP reduction in patients with ICH
- SBP b160 mm Hg in patients with aneurysmal SAH
- Patients achieving goal BP in 24 h: nicardipine, 100%; labetalol, 68% (P b .001)
- Patients achieving goal BP in 1 h: nicardipine, 88%; labetalol, 32% (P b .001)
- Time spent at goal BP over 24 h: nicardipine, 82.5%; labetalol, 48.5% (P b .001)
- Mean time to goal BP: nicardipine, 30 min; labetalol, 90 min (P = .026)
- Additional antihypertensive agents: nicardipine, 0%; labetalol, 73% (P b .001)
- Mean dose to achieve goal BP: nicardipine, 5 mg/h; labetalol, 50 mg
- Population: emergency department patients with hypertensive crises
- Study duration: 6 h
- Nicardipine dose: 5 mg/h titrated every 5 min by 2.5 mg/h until target reached or maximum of 15 mg/h
- Labetalol dose: 20 mg bolus over 2 min and then 20/ 40/80 mg bolus every 10 min until target reached or maximum of 300 mg
- Tx target: individualized target BP +- 20 mm Hg specified by treating physician before randomization of each patient
- Patients within target BP range in 30 min: nicardipine, 91.7%; labetalol, 82.5% (P = .039)
- Patients with 5 and 6 BP measurements within target range (of possible 6); nicardipine, 47.3%; labetalol, 32.8% (P = .026)
- (25/22)
Peacock et al [38] (110/116)
Varon et al [39] (52/52)
- Population: emergency department patients with renal failure and hypertension
- Patients within target range in 30 min: nicardipine, 92%; labetalol, 78% (P = .046)
- Study duration: 6 h o Patients with 5 and 6 BP measurements within target range (of possible 6): nicardipine, 46%; labetalol, 25% (P = .024)
Table 2 (continued)
Study (no. of nicardipine/labetalol patients)
Study design Results
- Nicardipine dose: 5 mg/h titrated every 5 min by 2.5 mg/h until target reached or maximum of 15 mg/h
- Labetalol dose: 20 mg bolus over 2 min and then 20/ 40/80 mg bolus every 10 min until target reached or max of 300 mg
- Tx target: individualized target BP +- 20 mm Hg specified by treating physician before randomization of each patient
 Malesker et al [40] o Population: critically ill patients o Patients who required a second antihypertensive agent:
Malesker et al [40] o Population: critically ill patients o Patients who required a second antihypertensive agent:
(159/159 c)
-
- Study duration: BP data for 36 h
- Mean nicardipine dose: 6.85 +- 1.75 mg/h
- Mean labetalol total dose: 203.8 +- 55.0 mg
- Tx target: NA
nicardipine, 34%; non- nicardipine, 59% (P b .001)
-
- Time to initiate oral antihypertensives: nicardipine,
19.7 h; non-nicardipine, 22.2 h (P b .001)
- Total hospital LOS: nicardipine, 174.6 h; non- nicardipine, 198.5 h (P b .001)
Kross et al [41] (20 d/22 d)
- Population: patients undergoing craniotomy o Patients who achieved target BP: nicardipine, 90%;
labetalol, 99% (P, NR)
-
- Study duration: 2 h (1 h before and 1 h after admission to the postanesthesia care unit)
- Incidence of treatment failure: nicardipine, 10 failures; labetalol, 4 failures (P = .05)
- Nicardipine dose: bolus doses at 2 mg/mL o Number of doses required to achieve target:
nicardipine, 5; labetalol, 11 (P, NR)
-
-
- Labetalol dose: bolus doses at 5 mg/mL
- Tx target: SBP b140 mm Hg
-
Elatrous et al [42] (30/30)
- Population: hypertension in pregnancy o Patients achieving target BP: nicardipine, 70%;
labetalol, 63% (P = .58)
- Study duration: 1 h o Time to reach target BP: nicardipine, 11.1 min; labetalol, 12.4 min (P, NS)
Thomas et al [43] (6/15)
- Nicardipine dose: 10 mg dose infused over 5 min increased to 12.5 mg over 5 min and then 15 mg/h if target BP not reached. At goal BP, maintenance dose of 1-3 mg/h started
- Labetalol dose: initial 1 min bolus of 1 mg/kg followed by 1.5 mg/kg after 5 min. At goal BP, maintenance dose of 100-150 mg/h started
- Tx target: 20% reduction in BP
- Population: pediatric patients aged <=24 mo with hypertensive crises or stage 2 hypertension (SBP N5 mm Hg above the 99th percentile)
- Mean no. of dosage adjustments required: nicardipine, 1.4; labetalol, 0.5 (P = .05)
- Time to reach target SBP +- SD: nicardipine, 5.8 +-
6.2 h; labetalol, 4.5 +- 5.5 h (P, NS) e
- Study duration: retrospective study o Time to reach target DBP +- SD: nicardipine, 4.4 +-
- Nicardipine mean starting dose: 0.5 +- 0.0 ug kg-1 min-1
- Labetalol mean starting dose: 0.44 +- 0.2 mg kg-1 h-1
- Tx target: 20% reduction in BP
6.2 h; labetalol, 4.3 +- 5.4 h (P, NS) e
- Hospital LOS +- SD: nicardipine, 116 +- 79 d; labetalol, 45 +- 53 d (P = .05) f
- ICU LOS +- SD: nicardipine, 45 +- 50 d; labetalol, 29 +- 35 d (P, NS) f
LOS indicates length of stay; NA, not applicable; NR, not recorded; NS, not significant; Tx, treatment.
a Nicardipine alone or after initial labetalol therapy-termed aggressive therapy.
b Labetalol alone termed standard therapy in this study.
c Treated with non-nicardipine antihypertensives-70 of these patients (44%) received labetalol.
d All patients were initially given IV enalaprilat 1.25 mg after closure of the dura mater.
e Calculations based on the number of Antihypertensive infusions (nicardipine, n = 9; labetalol, n = 21).
f Calculations based on the number of patients in each group (nicardipine, n = 6; labetalol, n = 15).
heterogeneity of treatments administered in the intensive care unit/emergency department was ascribed to a lack of comparative data and the absence of a consensus on the best IV agents to use in this patient population [4].
Labetalol and nicardipine are 2 antihypertensive agents that have been extensively used in the treatment of hypertensive crises for more than 20 years. The main characteristics of these drugs are shown in Table 1. Labetalol and nicardipine are recommended by the American Heart Association/American Stroke Association (AHA/ASA) as the initial IV treatment options for the management of severe hypertension after acute stroke [31,32]. A small number of head-to-head studies of nicardipine and labetalol have been performed across several different patient populations; however, to date, there has been no synthesis of these data. The authors recently developed predictive models using published data to compare the efficacy of nicardipine and labetalol when administered at Food and Drug Administration-recommended doses [33]. These models indicated that 45% to 61% of nicardipine-treated patients and 14% to 19% of labetalol-treated patients would achieve a clinically relevant BP drop within 30 minutes. The authors, therefore, concluded that the results of these models warranted a phase IV clinical trial to further examine the apparent treatment discrepancy between nicardipine and labetalol in the emergency treatment of hypertension. The current systematic review includes one such study, a randomized comparative effectiveness trial of IV nicardipine vs labetalol use in the emergency department (CLUE), and contextualizes its findings, alongside those of the predictive models, by formally analyzing the results of other published studies of nicardipine vs labetalol in the management of hypertensive crises.
Methods
A MEDLINE search was conducted on May 24, 2011, using the term “labetalol AND nicardipine.” No date limits were specified. Inclusion of studies was according to a predetermined set of criteria. To be included, studies had to be conducted with human subjects and be concerned with IV nicardipine and labetalol. Furthermore, for a study to be included in this review, nicardipine and labetalol could not be used in combination with each other or as part of complex sequential drug regimens. Preclinical studies, single-case studies, and studies not published in English were excluded from this review. In addition to the MEDLINE search, accepted congress abstracts concerning studies performed by the study’s authors were also included.
In total, 60 articles were identified by the MEDLINE search. Ultimately, 10 studies were included in the review. The flow of studies through the systematic review is illustrated in Fig. 1. Outcome data extracted from these studies to be reported in this review varied with indication and included the following: the magnitude of BP/mean arterial pressure (MAP) reductions, the proportion of patients
treated successfully according to the prespecified criteria of each study, frequency of adverse outcomes, hospital length of stay, time to initiation of oral antihypertensives, the number of dosage adjustments, and the use of additional IV antihypertensive agents.
Results
Of the 10 studies identified, the majority were concerned with hypertensive crises in patients with stroke (4 studies). The remaining studies were concerned with hypertension in varioUS settings including surgery, pediat- rics, pregnancy, the emergency department, and critically ill patients. The design and main results from each study are detailed in Table 2.
Stroke
It has been reported that up to 80% of the patients with acute ischemic stroke and 75% of those with intracerebral hemorrhage (ICH) have hypertension [44-46]. Acutely elevated BP is associated with adverse outcomes and poor responses to reperfusion therapy in patients with stroke [47-51], including increased rates of mortality, dependency, subsequent deterioration, Hematoma expansion, inComplete recovery, and an increased risk of recurrence [52-55]. In addition, BP variability after stroke is thought to be associated with more rapid Disease progression and worse outcome after recombinant tissue plasminogen activator (rt- PA) therapy [35,48,56-58]. It is, therefore, vital that hypertension is managed effectively and rapidly in this patient population [59].
Current AHA/ASA guidelines recommend treatment of elevated BP with IV antihypertensives in patients meeting the following criteria: SBP higher than 180 mm Hg or MAP higher than 130 mm Hg in patients with spontaneous ICH, SBP higher than 160 mm Hg in patients with unsecured Aneurysmal subarachnoid hemorrhage (SAH), SBP higher than 220 mm Hg/DBP higher than 120 mm Hg for patients with AIS who were not receiving thrombolytic therapy, or SBP higher than 185 mm Hg/DBP higher than 110 mm Hg for patients with AIS who were receiving thrombolytic therapy [31,32]. Both labetalol and nicardipine are recom- mended as First-line treatments for severe hypertension [31,32].
Liu-DeRyke and colleagues [35,37] have performed 2 comparative studies of nicardipine and labetalol in patients with hypertensive crises after ICH, AIS, or aneurysmal SAH. Both studies were conducted at a university-affiliated trauma center in Detroit, MI. Patients were eligible for inclusion in these studies if they required treatment of hypertension according to the AHA/ASA guidelines at the time of the study. Antihypertensive therapy was initiated in the emergency department in both studies.
The first study by Liu-DeRyke and colleagues [35] was a retrospective, nonrandomized investigation that evaluated MAP reduction and BP variability over the first 24 hours of hospitalization. Most patients in this study had experienced ICH (58% and 53% in the nicardipine and labetalol groups, respectively). Aneurysmal subarachnoid hemorrhage had been experienced by 23% and 22% of patients in the nicardipine and labetalol groups, respectively, and 19% and 25% had AIS. Patients in the labetalol group were significantly older than those in the nicardipine group (61 vs 55 years, respectively; P = .02). Most patients in both groups were women and African American. Nicardipine treatment resulted in less variable MAP reductions than those reported for patients given labetalol (Table 2; Fig. 2). Patients administered nicardipine were significantly more likely to achieve their target BP within 1 hour than patients given labetalol (P b .05). Furthermore, patients treated with nicardipine required fewer dosage adjustments or additional
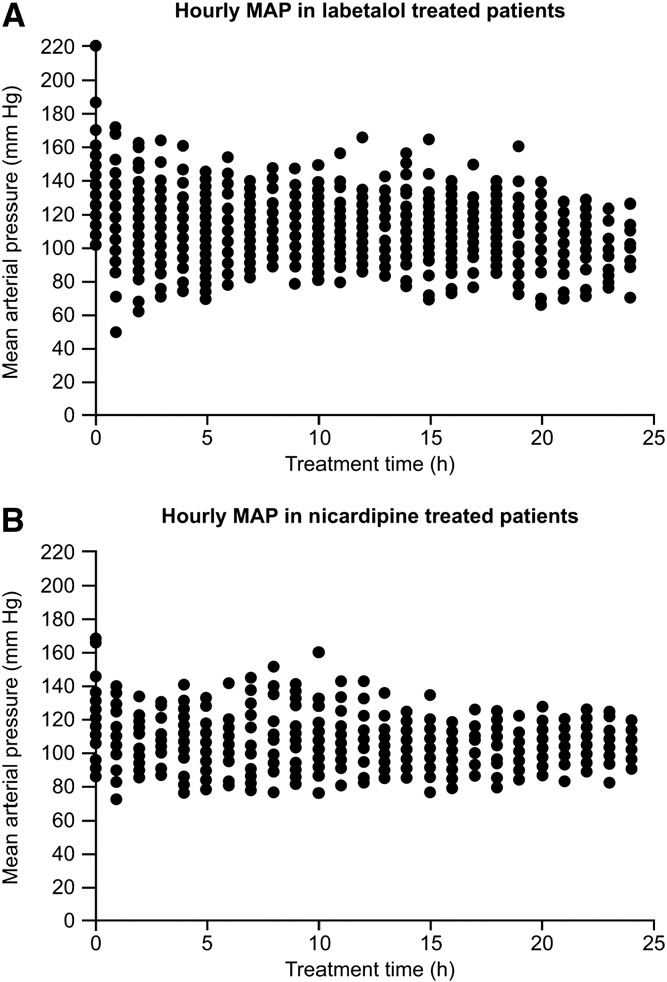
Fig. 2 Hourly MAP over 24 hours for labetalol-treated (A) and nicardipine-treated (B) patients [35]. Labetalol, n = 64 patients; nicardipine, n = 26 patients. Mean arterial pressure was recorded at hour 0 before administration of study medication. From Liu- DeRyke et al [35, Fig. 2], with kind permission from Springer Science and Business Media: Neurocrit Care.
antihypertensive agents than patients in the labetalol group (P b .05 for both; Table 2). Of the 5 nicardipine-treated and 19 labetalol-treated patients whose Hematoma volumes were measured at baseline and follow-up visits, 2 nicardipine- treated and 6 labetalol-treated patients experienced hemato- ma expansion. There were no significant differences in intensive care unit or hospital lengths of stay nor in patient disposition between the 2 treatment groups. Adverse event rates were similar for the 2 antihypertensive agents (Table 3). To expand and advance their original retrospective research, Liu-DeRyke and colleagues [37] designed a prospective, randomized clinical trial of patients with ICH, AIS, or SAH who received nicardipine or labetalol for BP management and evaluated their BP control over the first 24 hours. The study enrolled patients between August 2005 and July 2009. Patients were excluded if they had experienced brainstem herniation or traumatic brain injury or had a history of intracranial neoplasm, acute myocardial infarction, severe aortic stenosis, or bradycardia. Rescue medication with an additional IV antihypertensive agent was to be given to patients in the event of uncontrolled BP during the study period. Most patients in this study had experienced ICH (nicardipine group, 60%; labetalol group, 46%; P = .40). Nicardipine was significantly more effective at reducing BP in patients than labetalol (P b .001), according to all treatment parameters assessed (Table 2). None of the patients treated with nicardipine required rescue medication. Of the
16 labetalol-treated patients (72.7%) who were given an additional agent, 14 (87.5%) were treated with nicardipine, 4 (25.0%) received enalaprilat, 1 patient (6.3%) received hydralazine, and 1 patient (6.3%) received clonidine. All patients who received additional agents achieved their prespecified BP goal; the median time to reach target BP with rescue medication was 60 minutes. Significantly less BP variability occurred in patients treated with nicardipine compared with the labetalol group (Fig. 3). Adverse events were comparable in the 2 treatment groups (Table 3) [37].
Powers and colleagues [34] conducted a US-based prospective double-blind study of 14 patients with ICH who were randomized to receive either labetalol or nicardipine with a treatment goal to reduce their MAP by 15%. This study aimed to assess the effect of a prespecified reduction in BP on cerebral blood flow (CBF). Whole brain and periclot CBF were monitored using positron emission tomography at baseline and after stable MAP reduction. Average MAP at admission was 165 mm Hg in patients who received nicardipine and 150 mm Hg in those who received labetalol. The time the target MAP was achieved was not reported, although the patients in both treatment groups achieved the required reduction in MAP (Table 2). Mean
changes (+-SD) in global and periclot CBF were +0.19 +- 3.9 and +0.89 +- 3.2 mL 100 g-1 min-1, respectively, for nicardipine-treated patients and -1.55 +- 3.2 and -2.41 +- 2.7 mL 100 g-1 min-1, respectively, for labetalol-treated patients. Change in periclot CBF after reductions in MAP
showed a trend toward a difference between the nicardipine
Table 3 Safety results in included studies
Study (no. of nicardipine/ labetalol patients)
Powers et al [34] (7/7)
Liu-DeRyke et al [35] (26/64)
Martin-Schild et al [36] (26/24) Liu-DeRyke et al [37] (25/22)
Peacock et al [38] (110/116) Varon et al [39] (52/52) Malesker et al b [40] (159/159)
Kross et al [41] (20/22)
Elatrous et al [42] (30/30) Thomas et al [43] (6/15) d
No. of patients (%)
Nicardipine-treated patients NR
Bradycardia: 3 (12)
Hypotension: 0 (0)
Expansion of hematoma: 2/5 (13.3) a NR
Hypotension: 1 (4)
Bradycardia: 0 (0)
Tachycardia: 3 (12)
No adverse events observed No adverse events observed Arrhythmia: 12 (8) c
Hypotension: 16 (10)
Renal dysfunction: 9 (6)
Myocardial ischemia: 2 (1)
Bradycardia: 2 (10)
Tachycardia: 4 (20)
Hypotension: 3 (15)
Nausea: 1 (3)
Increased HR and palpitations: 3 (10)
Hypotension: 1 (11)
Bradycardia: 0 (0)
Hypoglycemia: 1 (11)
Labetalol-treated patients
NR
Bradycardia: 13 (20)
Hypotension: 2 (3)
Expansion of hematoma: 6/19 (17.6) a NR
Hypotension: 1 (5)
Bradycardia: 2 (9)
Tachycardia: 1 (5)
No adverse events observed No adverse events observed Arrhythmia: 29 (18)
Hypotension: 28 (18)
Renal dysfunction: 12 (8)
Myocardial ischemia: 13 (8)
Bradycardia: 2 (9)
Nausea: 1 (3)
Mild deceleration of fetal HR: 1 (3) Hypoglycemia: 2 (9.5)
Bradycardia: 1 (5)
Hypotension: 7 (33)
HR indicates heart rate; NR, not reported.
a All patients with ICH (labetalol, n = 34; nicardipine, n = 15).
b Comparator group was any non-nicardipine antihypertensive-most patients in this group (44%) received labetalol.
c P = .018 vs non-nicardipine group.
d Calculations based on the number of antihypertensive infusions (nicardipine, n = 9; labetalol, n = 21).
and labetalol groups (P = .06). However, labetalol-treated patients received more fentanyl than nicardipine-treated patients, and fentanyl dose was found to inversely correlate with CBF parameters. No trend was noted for a difference in
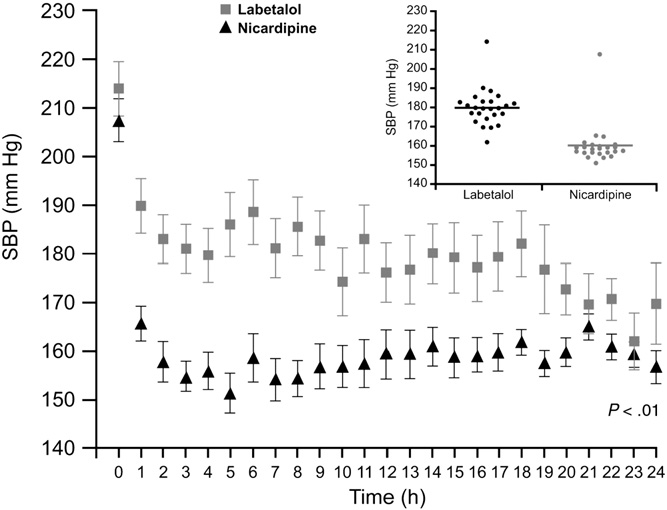
Fig. 3 Twenty-four-hour SBP control with nicardipine vs labetalol in patients with hypertensive crises after experiencing AIS, intracranial hemorrhage, or SAH [37]. Printed with permission from Dr. D.H. Rhoney.
periclot CBF between the nicardipine and labetalol groups after adjustment for fentanyl dose (P = .25).
Martin-Schild and colleagues [36] conducted a retrospec- tive, observational study of BP management in patients with AIS who presented to a single US emergency department between January 2004 and December 2006. Using medical record review, patients were identified who were given either “standard” or “aggressive” BP-lowering therapy so that they could be safely treated with rt-PA within 3 hours of stroke onset. Standard therapy comprised labetalol-a first-line agent for BP control before thrombolytic administration according to stroke treatment guidelines at the time [60]. Aggressive therapy was defined by choice of agents rather than target BP and comprised labetalol followed by nicardipine or nicardipine alone (nicardipine was first recommended as a first-line IV antihypertensive for thrombolysis-eligible patients with ICH in a later set of Stroke guidelines [61]). Initial SBPs in patients receiving standard and aggressive BP-lowering therapies were 195 and 206 mm Hg, respectively. Most patients who received BP- lowering therapy were men (57%) and had a history of hypertension (83%), although only 52% were receiving long-term Antihypertensive medication at baseline. Standard and aggressive BP-lowering therapies were equally effective (Table 2); however, patients treated with aggressive BP-
lowering therapy had a significantly shorter hospital length of stay than those given labetalol alone (Table 2). Rates of Hemorrhagic transformation, Neurologic deterioration, and mortality were similar for the 2 groups (P >= .2).
Emergency department
Hypertension is a common presentation in the emergency department, accounting for at least 3% of the more than 120 million annual visits in the United States [14,62,63]; however, comparative studies of IV antihypertensive agents are currently lacking in this setting.
As phase IV, multicenter, randomized comparative effectiveness study of nicardipine and labetalol, CLUE enrolled 226 patients from US emergency departments who had 2 SBP measurements greater than 180 mm Hg taken at least 10 minutes apart [38]. An individualized BP target
+-20 mm Hg was set for each patient by their treating physician before randomization in this study. Most patients enrolled were women (52.7%) and African American (76.4%); the average age was 53 years. Study subjects were followed for 6 hours or until discharged from the emergency department.
Overall, 63.3% of the patients had evidence of end-organ damage, and their initial median BP was 211/117 mm Hg. A significantly greater proportion of patients in the nicardipine group vs the labetalol group reached their target BP within 30 minutes (P = .039). This difference was maintained after adjustment for baseline variables, indicating that patients given nicardipine were more likely to be within target BP range at 30 minutes than patients given labetalol (odds ratio, 2.73; 95% confidence interval, 1.11-6.70; P = .028). Furthermore, patients receiving nicardipine had significantly higher rates of 5 and 6 measured BP values (of a possible 6 measurements) within the prespecified target range during the initial 30 minutes of therapy (Table 2). Changes in SBP with nicardipine and labetalol are shown in Fig. 4. The proportion of patients requiring rescue medication was similar between the 2 groups (nicardipine, 15.5% of patients; labetalol, 22.4%; P = .18). Patients treated with labetalol had a slower heart rate at all posttreatment measurements (P b
.01) compared with patients administered nicardipine, but at no point was the heart rate less than 70 beats/min. Patient disposition from the emergency department was similar for the 2 treatment groups; 46.8% and 57.4% of nicardipine- and labetalol-treated patients were discharged home (P = .11), whereas 49.5% and 38.3%, respectively (P = .089), were admitted to a hospital. No adverse events were reported for either agent during the study, although this may have been due to the brevity of the study duration [38].
A subanalysis in the CLUE study compared the efficacy and safety of nicardipine and labetalol in patients with renal failure (defined as creatinine clearance b75 mL/min) [39]. In this subpopulation, nicardipine-treated patients were more likely to reach their target BP within 30 minutes than those treated with labetalol. In addition, the nicardipine group was
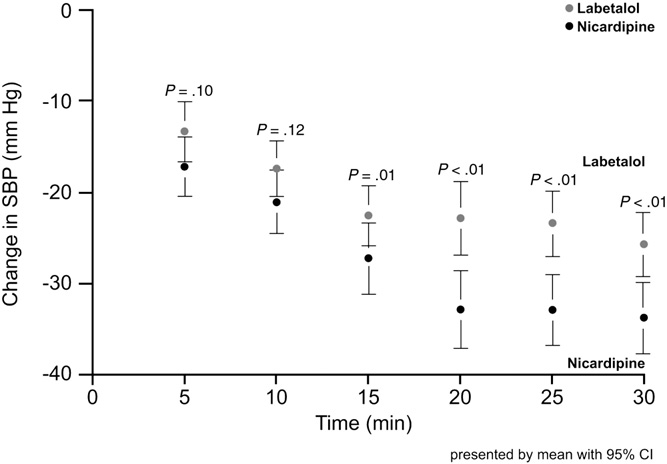
Fig. 4 Systolic BP changes with nicardipine and labetalol during the 30-minute study period [38]. CI indicates confidence interval. Printed with permission from Dr. W.F. Peacock.
more likely to have 5 or 6 BP measurements (of a possible 6 measurements) within the prespecified target range (Table 2). Adverse events were infrequent in this subanalysis (Table 3).
Intensive care unit
Malesker and colleagues [40] performed a retrospective study of 159 consecutive patients who received IV nicardipine at 2 hospital intensive care units in Omaha, NE, between January and December 2007. These patients were case matched with patients who received other IV antihypertensives. A large proportion of patients (44%) in the “other antihypertensives” group were given labetalol; hence, this study was included in the current systematic review. The duration of treatment, number of treatment adjustments, use of a second antihypertensive, time to initiation of oral antihypertensives, and length of hospital stay were compared between nicardipine and the other agents. In addition, an economic analysis was performed.
There were no significant differences in SBP or DBP during the 36-hour treatment with nicardipine vs other antihypertensives. However, the proportion of patients who required a second antihypertensive agent was significantly higher in the other antihypertensive group compared with the nicardipine-treated group (Table 2). Time to initiation of oral antihypertensives was significantly shorter in the nicardipine vs other antihypertensives group (P b .001; Table 2). Length of stay in the intensive care unit was longer with nicardipine compared with other agents (P b .01); however, the total length of hospital stay was significantly shorter with nicardipine (approximately 30 hours; Table 2). Table 3 shows the rates of complications recorded during treatment with antihypertensives. cardiac dysrhythmia was more common in the other antihypertensives group than in patients given nicardipine, the greatest proportion of which were bradycardia events that arose in patients treated with labetalol (17/29 of all dysrhythmic events and 17/20
bradycardic events). In most (88%) labetalol-treated patients who developed bradycardia, the drug had been administered as a bolus. Results of the economic analysis, adjusted for potential confounders, revealed that nicardipine use reduced the costs of hospital care by 17% compared with the other antihypertensive agents (P b .001).
Postoperative hypertension
Postoperative hypertensive crises occur in 4% to 35% of surgical patients [64-66]. Hypertension after emergence from anesthesia may result in bleeding and cerebral edema after craniotomy [67,68]. Preemptive treatment with antihyper- tensives is, therefore, imperative to avert these potentially life-threatening events.
Kross and colleagues [41] performed a randomized, open-label clinical trial to compare nicardipine and labetalol in patients with acute emergence hypertension after craniotomy for tumor surgery. They enrolled 42 patients between July 1995 and March 1999, none of whom had a diagnosis of hypertension before surgery. All patients were given IV enalaprilat 1.25 mg after the closure of the dura mater and before the administration of bolus doses of IV nicardipine or IV labetalol to maintain an SBP level lower than 140 mm Hg. Treatment failure was defined as SBP higher than 140 mm Hg for longer than 2 minutes. On closure of the dura mater, the mean (+-SD) SBP was 104 +- 9 and 109 +- 9 mm Hg in the labetalol and nicardipine groups, respectively. The combination of enalaprilat and labetalol was more effective at reducing BP compared with enalaprilat and nicardipine (Table 2). Adverse events were more common in the nicardipine- treated group compared with patients who were given labetalol (Table 3). One patient who received nicardipine experienced hypotension that required treatment with phenylephrine. Oxygen desaturation did not occur in any patient in either study group.
Hypertension during pregnancy
In pregnant women, hypertensive emergencies may occur at lower BPs than in the general clinical population (SBP N169 mm Hg or DBP N109 mm Hg). The physiology of pregnancy can lead to lower SBP and DBP, causing end- organ damage [9,10,12]. It has been reported that up to 7% of pregnant women will experience pregnancy-related hyper- tension (preeclampsia/eclampsia) [69]. Nicardipine IV has not been evaluated in adequate, well-controlled studies of pregnancy; thus, it is considered pregnancy category C and should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus [70].
Elatrous and others [42] conducted a prospective, randomized, single-blind study of nicardipine and labetalol for the management of acute hypertension in pregnancy. The study took place between January 1995 and December 1996
in the obstetric ward of a teaching hospital in Tunisia. Women 18 years or older were eligible for inclusion if they had passed 24 weeks of gestation and had 2 measurements repeated 30 minutes apart of an SBP level of 170 mm Hg or higher or a DBP level of 110 mm Hg or higher. At admission, patients who went on to be treated with nicardipine had a mean BP of 176/110 mm Hg; patients treated with labetalol had a Baseline BP of 171/110 mm Hg. Overall efficacy was comparable for nicardipine and labetalol (Table 2), although the decrease in BP was greater with nicardipine than with labetalol (P b .05). Although significantly more dosage adjustments were required with nicardipine, the authors noted that drug dosage was modified in very few cases with either agent. Rates of adverse events were similar for nicardipine and labetalol (Table 3). An increase (P b .01) in heart rate relative to baseline values was reported for 1 nicardipine-treated patient, although this did not lead to discontinuation of treatment. One episode of a mild, transient deceleration in fetal heart rate was reported in the labetalol group.
Hypertensive crises in the pediatric population
Hypertension is uncommon in the pediatric population, with a reported prevalence of 3.6% in children aged 3 to 18 years [71]. The prevalence of hypertensive crises in children is currently unknown. prompt treatment of hypertensive crises in children is essential; however, there are few data regarding the comparative efficacy and safety of agents in this patient population. It should be noted that the safety and efficacy of nicardipine in patients younger than 18 years has not been established [70].
Thomas and colleagues [43] conducted a retrospective chart review of pediatric patients (average age, ~360 days) admitted to a US children’s hospital between 2002 and 2008 who were treated with nicardipine, labetalol, or nitroprusside. Children 24 months or younger were eligible
for inclusion in this study if they had a discharge diagnosis of hypertensive crisis or stage 2 hypertension (defined as an SBP of N5 mm Hg above the 99th percentile). At baseline, patients who were treated with nicardipine had a mean SBP/DBP of 137/86 mm Hg, whereas patients treated with labetalol had a mean baseline SBP/DBP of 144/84 mm Hg. Nicardipine and labetalol demonstrated comparable efficacy in achieving a 20% reduction in patients’ BP (Table 2). Two patients were switched from nicardipine treatment to labetalol treatment (reasons not reported). The length of hospital stay for patients treated with nicardipine was significantly longer than that for labetalol-treated patients (P = .05), although the length of stay in the intensive care unit did not differ between the 2 agents (Table 2). The safety analysis in this study reported that 6 patients (40%) in the labetalol group died compared with 1 patient (17%) in the nicardipine group (P, not significant). Differences in the rates of adverse events (hypotension, bradycardia, and hypoglycemia) for patients
treated with nicardipine and labetalol did not reach statis- tical significance (Table 3). However, labetalol infusions were discontinued prematurely (within 8 hours) in 6 patients due to hypotension; 5 of these patients had ischemic or traumatic brain injury, and 1 patient had symptoms of hypertensive encephalopathy. Caution may, therefore, be necessary when using labetalol to treat patients with brain injuries who have hypertensive crises [43].
Discussion
The varying end points and clinical populations in the studies reviewed here make it difficult to draw firm conclusions with regard to the comparative efficacy and safety of nicardipine vs labetalol. Indeed, there is a need for further research, as data are lacking from studies that measure long-term outcomes in patients experiencing hypertensive crises.
The study investigators of the trials discussed herein concluded that nicardipine and labetalol had comparable efficacy for the treatment of hypertensive crises and that nicardipine was an acceptable alternative to labetalol for the treatment of hypertension in the various patient populations. Overall, nicardipine provided more consistent and predictable control of BP than labetalol, with a similar rate of complications. The safety profiles of nicardipine and labetalol were comparable in the reviewed studies. Hypotension and arrhythmia were the most commonly reported adverse events (hypotension was reported in up to 15% of nicardipine-treated patients vs up to 18% of labetalol-treated patients; arrhythmia was reported in up to 20% of nicardipine- and labetalol-treated patients).
A number of other Clinically relevant findings were also reported in these studies. For example, in critically ill patients, nicardipine was associated with lower overall Costs of care than labetalol [40]. The study investigators suggested that the higher costs of care associated with labetalol treatment were due to the use of additional agents, the extended use of IV vs oral antihypertensives, and the cost of treating complications such as bradycardia. In response to concerns regarding iatrogenic end-organ damage in patients experiencing severe hypertension, reduction of MAP with nicardipine or labetalol in patients with ICH did not reduce CBF and was, therefore, unlikely to lead to hypoperfusion in patients with stroke [34].
One theme reiterated in a number of the studies was the relative lack of awareness among health care professionals regarding the use of nicardipine in patients with stroke, despite its inclusion in AHA/ASA guidelines [32,36]. Potential problems due to inexperience with nicardipine were reported in a study of surgical patients [41], the results of which showed that nicardipine treatment resulted in more adverse events and poorer control of BP [41]. Such differences, however, were felt by study authors to possibly
reflect inadequate dosing of nicardipine resulting from clinician unfamiliarity with the drug itself [41].
Issues with the time required to prepare a nicardipine infusion have also been mentioned. One group of study investigators [36] stated that the longer time between admission and treatment with rt-PA with nicardipine vs labetalol was due to the time taken to mix and set up an infusion system for nicardipine, as well as tentative early dosing with this agent. In contrast, a labetalol bolus was readily available and could, therefore, be administered immediately. This concern was also reported in a recent study of antihypertensive agents for the control of BP in patients after ICH, which noted that slow pharmacy delivery of antihypertensive medications was a major reason for the delay in achieving the target MAP [72]. The introduction of ready-mixed nicardipine may reduce this delay substantially and could potentially improve Treatment outcomes in this indication as a result. Indeed, many of the patients randomized to receive nicardipine in the prospective study performed by Liu-DeRyke and colleagues [37] received the ready-to-use formulation and achieved better BP control than did patients treated with labetalol.
There is currently increased interest in the appropriate management of acute severe hypertension after ICH due to the findings of the Antihypertensive treatment of Acute Cerebral Hemorrhage and Intensive Blood pressure reduction in Acute Cerebral Haemorrhage Trial studies [73-76]. The results of these studies suggest that clinical outcomes in patients with severe hypertension after ICH may be improved by BP reductions with IV antihyperten- sive agents to SBP less than 140 mm Hg compared with the standard SBP reduction recommended by AHA/ASA guidelines (b180 mm Hg [32]). In the relatively small Antihypertensive Treatment of Acute Cerebral Hemorrhage study, improvements in patients whose SBP was main- tained at less than 140 mm Hg included reduced rates of hematoma expansion and better 3-month outcome, espe- cially if treated within 3 hours of symptom onset [73,74,76]. Determining the most appropriate target BP and the specific agents to use for rapid, controlled reductions in BP is, therefore, a research priority.
Conclusion
In conclusion, studies published to date indicate that nicardipine and labetalol have comparable efficacy for the treatment of hypertensive crises across a number of challenging patient populations. The BP control achieved with nicardipine is more consistent than with labetalol, whereas the adverse event profiles of these agents are similar. At present, there appears to be a requirement for education regarding the appropriate use and the approved indications for nicardipine. In addition, further prospective comparative studies of longer duration and in larger sample sizes are required to assess whether Clinically meaningful long-term
treatment benefits may exist with the use of one agent over the other.
References
- Vidt DG. Current concepts in treatment of hypertensive emergencies. Am Heart J 1986;111:220-5.
- McRae RPJ, Liebson PR. Hypertensive crisis. Med Clin North Am 1986;70:749-67.
- Chobanian AV, Bakris GL, Black H, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:2106-52.
- Katz JN, Gore JM, Amin A, et al. Practice patterns, outcomes, and end- organ dysfunction for patients with acute severe hypertension: the Studying the Treatment of Acute hyperTension (STAT) Registry. Am Heart J 2009;158:599-606.
- Ault MJ, Ellrodt AG. Pathophysiological events leading to the end- organ effects of acute hypertension. Am J Emerg Med 1985;3:10-5.
- Wallach R, Karp RB, Reves JG. Pathogenesis of paroxysmal hypertension developing during and after coronary bypass surgery: a study of hemodynamic and humoral factors. Am J Cardiol 1980;46: 559-65.
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet 2000;356:
411-7.
- Chatzizisis YS, Coskun AU, Jonas M, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular and vascular behavior. J Am Coll Cardiol 2007;49:2379-93.
- Varon J. Treatment of acute hypertension: current and newer agents. Drugs 2008;68:283-97.
- Marik PE, Varon J. Hypertensive crises: challenges and management. Chest 2007;131:1949-62.
- Varon J, Marik PE. The diagnosis and management of hypertensive crises. Chest 2000;118:214-27.
- Varon J, Marik PE. Clinical review: the management of hypertensive crises. Crit Care 2003;7:374-84.
- Martin JFV, Higashiama E, Garcia E, et al. Hypertensive crisis profile: prevalence and clinical presentation. Arq Bras Cardiol 2004;83:131-6.
- Zampaglione B, Pascale C, Marchisio M, et al. Hypertensive urgencies and emergencies: prevalence and clinical presentation. Hypertension 1996;27:144-7.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997;350:757-64.
- Potter JF. Malignant hypertension in the elderly. Q J Med 1995;88: 641-7.
- Bennett SM, Shea S. Hypertensive emergency: case criteria, socio- demographic profile, and previous care of 100 cases. Am J Public Health 1988;78:636-40.
- Karras DJ, Kruus LK, Cienki JJ, et al. Evaluation and treatment of patients with severely Elevated blood pressure in academic emergency departments: a multicenter study. Ann Emerg Med 2006;47:230-6.
- Lund-Johansen P. Pharmacology of combined alpha-beta-blockade. II. Haemodynamic effects of labetalol. Drugs 1984;28(Suppl 2):35-50.
- Kaplan JA. Clinical considerations for the use of intravenous nicardipine in the treatment of postoperative hypertension. Am Heart J 1990;119:443-6.
- Lambert CR, Hill JA, Nichols WW, et al. Coronary and systemic hemodynamic effects of nicardipine. Am J Cardiol 1985;55:652-6.
- Vincent JL, Berlot G, Presier JC, et al. Intravenous nicardipine in the treatment of postoperative Arterial hypertension. J Cardiothorac Vasc Anesth 1997;11:160-4.
- Sabbatini M, Strocchi P, Amenta F. Nicardipine and treatment of cerebrovascular diseases with particular reference to hypertension- related disorders. Clin Exp Hypertens 1995;17:719-50.
- Wallin JD. Adrenoreceptors and renal function. J Clin Hypertens 1985;1:171-8.
- Marx PG, Reid DS. Labetalol infusion in acute myocardial infarction with systemic hypertension. Br J Clin Pharmacol 1979;8:233S-8S.
- Pearce CJ, Wallin JD. Labetalol and other agents that block both alpha- and beta-adrenergic receptors. Cleve Clin J Med 1994;61:59-69.
- Olsen KS, Svendsen LB, Larsen FS, et al. Effect of labetalol on cerebral blood flow, Oxygen metabolism and autoregulation in healthy humans. Br J Anaesth 1995;75:51-4.
- Kitiyakara C, Guzman NJ. Malignant hypertension and hypertensive emergencies. J Am Soc Nephrol 1998;9:133-42.
- Varon J. The diagnosis and treatment of hypertensive crises. Postgrad Med 2009;121:5-13.
- Kanto J, Allonen H, Kleimola T, et al. Pharmacokinetics of labetalol in healthy volunteers. Int J Clin Pharmacol Ther Toxicol 1981;19:41-4.
- Adams H, Adams R, Del Zoppo G, et al. Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update. A scientific statement from the Stroke Council of the American Heart Association/American Stroke Association. Stroke 2005;36:916-23.
- Broderick J, Connolly S, Feldman E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update. A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation 2007;116:e391-413.
- Peacock WF, Liang E, Kwei L, et al. Modeling comparative effectiveness studies: an example using a phase IV intravenous nicardipine versus labetalol in patients with Uncontrolled hypertension trial. J Pharmacokinet Pharmacodyn (submitted).
- Powers WJ, Zazulia AR, Videen TO, et al. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology 2001;57:18-24.
- Liu-DeRyke X, Janisse J, Coplin WM, et al. A comparison of nicardipine and labetalol for acute hypertension management follow- ing stroke. Neurocrit Care 2008;9:167-76.
- Martin-Schild S, Hallevi H, Allbright KC, et al. Aggressive blood pressure-lowering treatment before tissue plasminogen activator therapy in acute ischemic stroke. Arch Neurol 2008;65:1174-8.
- Liu-DeRyke X, Parker Jr D, Levy P, et al. A prospective evaluation of labetalol vs nicardipine for blood pressure management in patients with acute stroke. Crit Care Med 2009;37(Suppl):A161 [Abstract No. 342].
- Peacock WF, Baumann B, Borczuk P, et al. CLUE: a randomized comparative effectiveness trial of IV nicardipine versus labetalol use in the emergency department. Crit Care (in press).
- Varon J, Baumann BM, Cannon C, et al. A comparative effectiveness trial of intravenous (IV) nicardipine versus labetalol in patients with hypertensive crises and renal dysfunction. Submitted abstract to be presented at the Chest Annual Meeting, October 29-November 4, 2010, Vancouver, Canada. Chest 2010;138:906A.
- Malesker MA, Kondrack RR, Hilleman DE. Nicardipine in the management of hypertension in critically ill patients. Poster presented at the Chest Annual Meeting, October 31-November 5, 2009, San Diego, CA, USA. Chest Meeting Abstracts, 136; 2009. p. 43s-a.
- Kross RA, Ferri E, Leung D, et al. A comparative study between a calcium channel blocker (nicardipine) and a combined ?-?-blocker (labetalol) for the control of emergence hypertension during craniotomy for tumor surgery. Anesth Analg 2000;91:904-9.
- Elatrous S, Nouira S, Ouanes BL, et al. Short-term treatment of severe hypertension of pregnancy: prospective comparison of nicardipine and labetalol. Intensive Care Med 2002;28:1281-6.
- Thomas CA, Moffett BS, Wagner JL, et al. Safety and efficacy of intravenous labetalol for hypertensive crisis in infants and small children. Pediatr Crit Care Med 2011;12:28-32.
- Wallace JD, Levy LL. Blood pressure after stroke. JAMA 1981;246: 2177-80.
- Britton M, Carlsson A, de Faire U. Blood pressure course in patients with acute stroke and matched controls. Stroke 1986;17:861-4.
- Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients presenting to the emergency department with stroke in the United States. Am J Emerg Med 2007;25:32-8.
- Ahmed N, Wahlgren N, Brainin M, et al. Relationship of blood pressure, antihypertensive therapy and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International stroke thrombolysis Register (SITS-ISTR). Stroke 2009;40:2442-9.
- Delgado-Mederos R, Ribo M, Rovira A, et al. Prognostic significance of Blood pressure variability after thrombolysis in acute stroke. Neurology 2008;71:552-8.
- Katzan IL, Hammer MD, Furlan AJ, et al. Quality improvement and tissue-type plasminogen activator for acute ischemic stroke: a Cleveland update. Stroke 2003;34:799-800.
- Nogueira RG, Liebeskind DS, Sung G, et al. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: Pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI trials. Stroke 2009;40:3777-83.
- Shin HK, Nishimura M, Jones PB, et al. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke 2008;39:1548-55.
- Chamorro A, Vila N, Ascaso C, et al. Blood pressure and functional recovery in acute ischemic stroke. Stroke 1998;29:1850-3.
- Fogelholm R, Avikainen S, Murros K. Prognostic value and determinants of first-day mean arterial pressure in spontaneous supratentorial intracerebral hemorrhage. Stroke 1997;28:1396-400.
- Qureshi AI, Harris-Lane P, Kirmani JF, et al. Treatment of acute hypertension in patients with intracerebral hemorrhage using Amer- ican Heart Association Guidelines. Crit Care Med 2006;34:1975-80.
- Willmot M, Leonardi-Bee J, Bath PMW. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004;43:18-24.
- Geeganage CM, Bath PM. Relationship between therapeutic changes in blood pressure and outcomes in acute stroke: a metaregression. Hypertension 2009;54:775-81.
- Leira R, Millan M, Diez-Tejedor E, et al. Age determines the effects of blood pressure lowering during the acute phase of ischemic stroke: the TICA study. Hypertension 2009;54:769-74.
- Sare GM, Ali M, Shuaib A, et al. Relationship between hyperacute blood pressure and outcome after ischemic stroke: data from the VISTA collaboration. Stroke 2009;40:2098-103.
- Varon J. Diagnosis and management of Labile blood pressure during acute Cerebrovascular accidents and other antihypertensive crises. Am J Emerg Med 2007;25:949-59.
- Adams Jr HP, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke: a scientific statement from the Stroke Council of the American Stroke Association. Stroke 2003;34:1056-83.
- Adams Jr HP, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke. Circulation 2007;115: e478-534.
- Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data 2007(386):1-32.
- Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report 2008(7):1-38.
- Prys-Rroberts C. Anesthesia and hypertension. Br J Anaesth 1984;56: 711-24.
- Gal TJ, Cooperman LH. Hypertension in the immediate postoperative period. Br J Anaesth 1975;47:70-4.
- Halpern NA, Alicea M, Krakoff LR, et al. Postoperative hypertension: a prospective, placebo-controlled, randomized, double-blind trial, with intravenous nicardipine hydrochloride. Angiology 1990;41:992-1004.
- Kalfas IH, Little JR. Postoperative hemorrhage: a survey of 4992 intracranial procedures. Neurosurgery 1988;23:343-7.
- Fukumachi A, Koizumi H, Nukui H. Postoperative intracerebral hemorrhages: a survey of computed tomographic findings after 1074 intracranial operations. Surg Neurol 1985;23:575-80.
- Sibai BM. Preeclampsia-eclampsia. Curr Probl Obstet Gynecol Infert 1990;13:3-45.
- Cardene IV [package insert]. Bedminster (NJ): EKR Therapeutics, Inc.; 2007. Available at: http://www.cardeneiv.com/pdf/20mg_PI.pdf. Last accessed: May 26, 2010.
- Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA 2007;298:874-9.
- Honner SK, Singh A, Cheung PT, et al. Emergency department control of blood pressure in intracerebral hemorrhage. J Emerg Med 2009 [Epub ahead of print].
- Anderson CS, Huang Y, Wang JG, et al. Intensive blood pressure reduction in acute cerebral hemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol 2008;7:391-9.
- Anderson CS, Huang Y, Arima H, et al. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT). Stroke 2010;41:307-12.
- ATACH investigators: antihypertensive treatment of acute cerebral hemorrhage. Crit Care Med 2010;38:637-48.
- Qureshi AI, Palesch YY, Martin R, et al. Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage: results from the antihypertensive treatment of acute cerebral hemorrhage study. Arch Neurol 2010;67:570-6.
