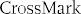An ultrasound training program’s effect on central venous catheter locations and complications
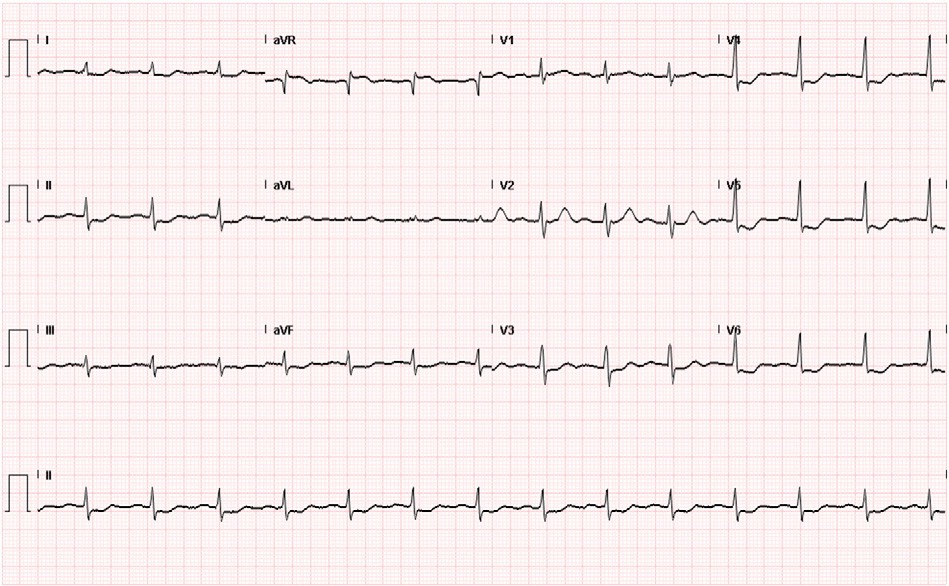
Fig. 1. Electrocardiogram on admission to the ED showing diffuse STD and STE in aVR N V1.
ultrasound training program’s effecto”>Fig. 2. Coronary angiography showing 99% left main occlusion (A), followed by Thrombolysis In Myocardial Infarction 3 flow after left main stenting (B).
Kosuge M, Ebina T, Hibi K, Morita S, Endo M, Maejima N, et al. An early and simple predictor of severe left main and/or three-vessel disease in patients with non- ST-segment elevation acute coronary syndrome. Am J Cardiol 2011;107(4):495-500.
An ultrasound training program’s effect on central venous catheter locations and complications?,??

To the Editor,
Central venous catheter placement is frequently performed in emergency department (ED) patients. Multiple studies have shown that ultrasound-guided (USG) Central venous catheter place- ment decreases overall mechanical complication rate, number of attempts, and time to cannulation for the internal jugular (IJ) and
? Meetings: American College of Emergency Physicians (ACEP) Research Forum, Denver, CO-October 2012. American Institute of Ultrasound in Medicine (AIUM) Annual Convention, New York, NY-April 2013.
?? Grant: None.
femoral vein locations [1-9]. The Agency for Healthcare Research and Quality recommends the use of USG for CVC placement [10], Consequentially, we postulated that the subclavian site may be less preferred for CVCs, as the data supporting USG at this location are not as abundant or conclusive [1,11-16].
In light of evidence supporting USG CVC placement, emergency medicine residents are trained in this procedure. Recent studies demonstrated that residents’ skills in CVC placement improved after instruction [17,18,19]. To date, no studies have shown how imple- mentation of an Emergency Department Ultrasound Training (EDUST) program, with formalized training in USG CVCs influences selection patterns of emergency physicians when choosing the site for CVCs and how these patterns impact procedural complication rates. The primary outcome of this investigation was to assess location selection patterns of CVCs before and after establishment of an EDUST. A secondary outcome was to identify and compare mechanical complication rates before and after the EDUST.
We retrospectively reviewed all CVCs placed pre-EDUST and post- EDUST in a tertiary care hospital. The study was approved by the institutional review board. We queried the ED electronic medical record for all CVCs placed between July 1, 2004, and June 30, 2007 (pre-EDUST) and July 1, 2007 and June 30, 2010 (post-EDUST). As part of the EDUST, all ED interns received lectures related to bedside ultrasonography, including USG CVC placement and the opportunity to practice on
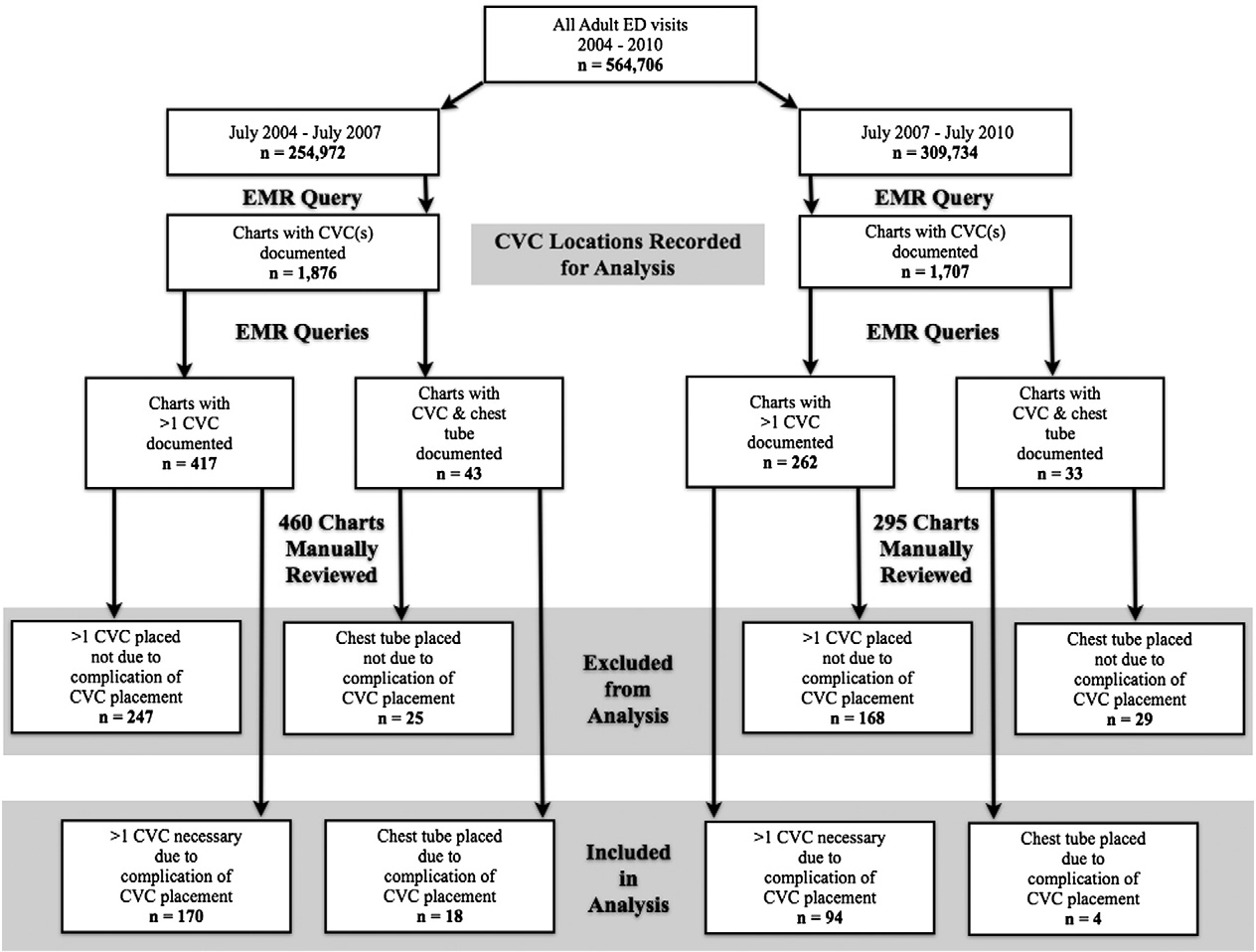
Fig. 1. Study algorithm.
vascular phantoms. In addition, a month-long ultrasound rotation was mandated for first and second year residents in which concepts underpinning USG CVC techniques were reviewed. Residents further developed their sonographic skillset during scanning shifts. Clinically, residents placed USG CVCs under supervision of a credentialed attending. All patients, older than 18 years old who received a CVC between 2004 and 2010 had their charts queried to select for those with more than 1 CVC documented. A second query looked for charts with both a CVC and tube thoracostomy documented. These criteria served as Surrogate markers for CVC mechanical complications. Charts were then manually reviewed to ensure that the second CVC was a result of a complication of the first CVC and that the tube thoracostomy was
placed as a result of a complication from the CVC (Fig. 1).
To ensure Interrater agreement, abstractors were trained on a protocol for chart review and met weekly to review a random sampling of charts. Location of CVCs and associated complications were noted in
data collection sheets. Mechanical complications included were inability to cannulate vessel, inability to thread guide wire, pneumo- thorax, tube thoracostomy, Arterial puncture, hemothorax, and improper catheter tip location. Catheter malposition was defined as any tip position seen on chest x-ray prompting removal and replacement with a second catheter. Location patterns and complica- tions were compared in the periods before and after the EDUST.
Patient characteristics for both study periods are summarized in Table 1. There was a significant change in CVC location selection patterns after implementation of an EDUST, with a sharp increase in utilization of the IJ site and dramatic reduction in selection of the subclavian site (Fig. 2 and Table 2). Overall mechanical complication rate decreased from 9.1% to 5.4%. Specific complication rates are outlined in Table 3. A Cohen’s ? coefficient of 0.82 (P b .0001) was calculated, signifying excellent agreement between abstractors based on random sampling of 20% of charts.
Table 1
Characteristics of study subjects
|
Patient characteristics |
Overall |
July 2004 to June 2007 |
July 2007 to June 2010 |
P |
|
564 706 |
254 972 |
309 734 |
- |
|
|
Central lines placed |
3583 |
1876 |
1707 |
- |
|
Average age |
75 |
76 |
74 |
.68 |
|
Average emergency severity index |
1.68 |
1.68 |
1.67 |
.94 |
|
Admitted to ICU |
89 |
62 |
27 |
.21 |
|
Died |
33 |
14 |
19 |
.002 |
|
Cardiac arrest |
41 |
16 |
25 |
b.0001 |
Abbreviation: ICU, intensive care unit.
July 2004 - June 2007 July 2007 - June 2010
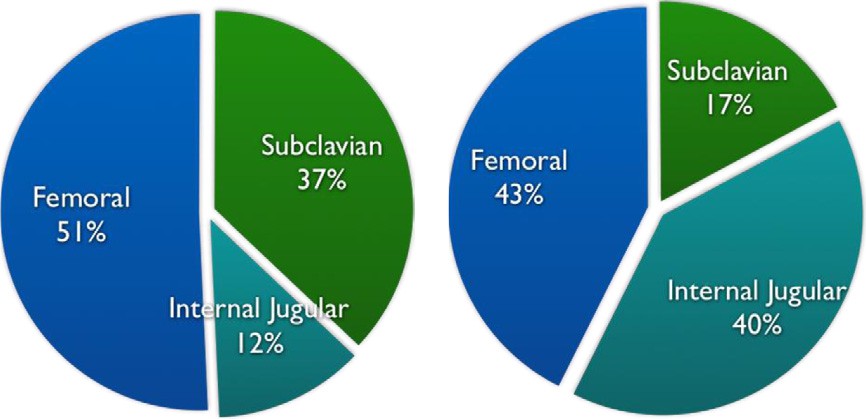
Fig. 2. CVC locations before and after EDUST.
During the 3-year period after the EDUST, our ED saw an 8% census increase with a 9% decrease in CVCs placed, whereas overall CVC complication rate decreased by 41%. In addition, there was a marked change of clinical practice in our ED, with the IJ site selected considerably more often than the subclavian site post-EDUST. Although we demonstrate a strong temporal association between implementation of EDUST, location pattern changes, and decreased complication rates, we cannot state that EDUST was the direct cause. Reported rate of pneumothorax from subclavian catheters ranges from 1.5% to 3.1%, whereas the rate from IJ catheters ranges from 0.1% to 0.2% [20,21,22]. These complication rates were reflected in our findings. Similarly, previous studies have shown a decrease in arterial puncture with use of USG [2,5-7,11], which corresponds to our 52% decrease in arterial puncture rates. In addition, there was a 45% decrease in rate of unsuccessful vein cannulation. This is important because increased number of attempts corresponds with increased complication rates [12,14]. Although there were more study patients who died after implementation of EDUST, we found that all the
cardiac arrests, except one, occurred before CVC placement.
This study suggests that implementation of a formal EDUST leads to a change in selection pattern for CVC placement sites. Complication rates also significantly decreased, demonstrating a direct impact on Patient care and safety.
Tahisha Nicole Tolbert, MD* Lawrence E. Haines, MD, MPH Victoria Terentiev, BA
Lucas McArthur, MD Antonios Likourezos, MA, MPH
Peter Homel, PhD Corey Weiner, MD Eitan Dickman, MD
Department of Emergency Medicine Maimonides Medical Center, Brooklyn, NY USA
*Corresponding author. 150 4th Ave no. 2A, Brooklyn, NY 11217.
E-mail addresses: [email protected];[email protected] http://dx.doi.org/10.1016/j.ajem.2014.07.025
Table 2
Central venous catheter site selection patterns
References
- Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med 1996;24:2053-8.
- Leung J, Duffy M, Finckh A. Real-time ultrasonographically-guided internal jugular vein catheterization in the emergency department increases success rates and reduces complications: a randomized, prospective study. Ann Emerg Med 2006;48:540-6.
- Miller AH, Roth BA, Mills TJ, Woody JR, Longmoor CE, Foster B, et al. Ultrasound guidance versus the landmark technique for the placement of central venous catheters in the emergency department. Acad Emerg Med 2002;9:800-5.
- Slama M, Novara A, Safavian A, Ossart M, Safar M, Fagon JY, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med 1997;23:916-9.
- Denys BG, Uretsky BF, Sudhakar R. Ultrasound-assisted cannulation of the internal jugular vein. Circulation 1993;87:1557-62.
- Hilty WM, Hudson PA, Levitt MA, Hall JB. Real-time ultrasound-guided femoral vein catheterization during cardiopulmonary resuscitation. Ann Emerg Med 1997; 29:331-7.
- Wu SY, Ling Q, Cao LH, Wang J, Xu MX, Zeng WA, et al. Real-time two dimensional ultrasound guidance for central venous cannulation. Anesthesiology 2013;118: 361-75.
- Karakitsos D, Labropoulos N, DeGroot E, Patrianakos AP, Kouraklis G, Poularas J, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care 2006;10:1-8.
- Turker G, Kaya FN, Gurbert A, Aksu H, Erdogan C, Atlas A, et al. Internal jugular vein cannulation: an ultrasound-guided technique versus a landmark-guided tech- nique. Clinics (Sao Paulo) 2009;64:989-92.
- Agency for Healthcare Research and Quality. Evidence report/technology assessment, no. 43: making health care safer: a critical analysis of patient safety practices: chapter 21 ultrasound guidance of central vein catheteriza- tion. Available at http://www.ahrq.gov/clinic/ptsafety. [AHRQ Publication No. 01E058. Accessed March 1, 2013].
- Fragou M, Gravvanis A, Dimitriou V, Papalois A, Kouraklis G, Karabinis A, et al. Real- time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med 2011;39: 1607-12.
- Mansfield PF, Hohn DC, Fornage BD, Gregurich MA, Ota DM. Complication and failures of subclavian-vein catheterization. N Engl J Med 1994;331:1735-8.
- Griswold-Theodorson S, Farabaugh E, Handly N, McGrath T, Wagner D. Subclavian central venous catheters and ultrasound guidance: policy vs practice. J Vasc Access 2012;21:1-7.
- Hind D, Calvert N, McWilliams R, Davidson A, Paisley S, Beverley C, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ 2003;327: 1-7.
- Lefrant JY, Cuvillon J, Benezet JF, Dauzat M, Peray P, Saissi G, et al. Pulsed Doppler ultrasonography guidance for catheterization of the subclavian vein: a random- ized study. Anesthesiology 1998;88:1195-201.
- Bold RJ, Winchester DJ, Madary AR, Gregurich MA, Mansfield PF. Prospective, randomized trial of Doppler-assisted subclavian vein catheterization. Arch Surg 1998;133:1089-93.
CVC site July 2004 to
June 2007
July 2007 to
June 2010
Absolute % difference
95% CI P
Woo MY, Frank J, Lee CA, Thompson C, Cardinal P, Yeung M, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound-guided insertion of central venous catheters. CJEM 2009;11:343-8.
Subclavian 37.0% 17.3% 19.7% 17.1%-22.8% b.0001
IJ 12.2% 40.2% 28.0% 25.0%-30.8% b.0001
Femoral 50.8% 42.5% 8.3% 4.7%-11.3% b.0001
Abbreviation: CI, confidence interval.
Evans LV, Dodge KL, Shah TD, Kaplan LJ, Siegel MD, Moore CL, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med 2010;85:1462-9.
- Shokoohi H, Boniface K, McCarthy M, Khedir Al-tiae T, Sattarian M, Ding R, et al. Ultrasound-guided peripheral intravenous access program is associated with a
Table 3
Central venous catheter complications
Complications
July 2004 to June 2007
July 2007 to June 2010
Absolute % difference
95% CI
P
Overall
171 (9.1%)
92 (5.4%)
3.7%
1.94%-5.42%
.001
Pneumothorax
23 (1.2%)
6 (0.4%)
0.8%
0.25%-1.55%
.004
Tube thoracostomy
18 (1%)
4 (0.2%)
0.8%
0.17%-1.33%
.005
Arterial puncture
47 (2.5%)
21 (1.2%)
1.3%
0.38%-2.19%
.005
Unsuccessful attempt
160 (8.5%)
80 (4.7%)
3.8%
2.22%-5.47%
.032
Unable to thread
4 (0.2%)
0 (0%)
0.2%
-0.05% to 0.55%
.306
Hemothorax
1 (0.05%)
1 (0.06%)
0.01%
-0.25% to 0.28%
.726
Catheter tip malposition
12 (0.6%)
11 (0.6)
0%
-
.574
marked reduction in central venous catheter use in noncritically ill emergency department patients. Ann Emerg Med 2013;61:198-203.
Department of Health and Human Services. Medicare learning network, October 2012. hospital-acquired conditions in acute inpatient prospective payment system hospitals. Available at http://www.cms.gov/Medicare/Medicare-Fee-for-Service- payment/HospitalAcqCond/downloads/hacfactsheet.pdf. [Accessed April 3, 2013].
- McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med 2003;348:1123-33.
- Merrer J, De Jonghe B, Golliot F, Lefrant JY, Raffy B, Barre E, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 2001;286:700-7.
Valsalva retinopathy and branch retinal artery occlusion after cardiopulmonary cerebral resuscitation


To the Editor,
We have read the case report “Valsalva retinopathy and branch retinal artery occlusion after cardiopulmonary cerebral resuscitation” with great interest [1]. They aimed to present a rare case of Valsalva retinopathy and subsequent branch retinal arteriole occlusion (BRAO) after practicing cardiopulmonary cerebral resuscitation. They concluded that optic nerve located hemorrhage may evoked the disc edema and this leads to BRAO or an emboli from patent foramen ovale may have caused BRAO.
Branch retinal arteriole occlusion is a sight and visual field treating condition. There is segmental retinal infarction in the region supplied by the occluded branch retinal arteriole. Systemic conditions that contribute to embolus formation such as diabetes mellitus; Arterial hypertension; ischemic heart diseases; and ocular conditions, including prepapillary arterial loops, optic disc drusen, increased Intraocular pressure, toxoplasmosis, and Optic neuritis, can lead to BRAO [2]. Carotid embolus formation and carotid dissection can also be associated with BRAO [3,4]. It is recommended that carotid artery Doppler ultrasonography should be performed in retinal artery occlusion patients [5]. It is not mentioned that carotid examination was performed. In this patient, carotid artery Doppler ultrasonography should be performed to rule out carotid artery embolus formation, carotid artery dissection should also be kept in mind, and Carotid ultrasonography should also be performed.
In the article, the authors declare that the patient has come to their clinic on the 10th day, but they provided fundus photo immediately after cardiopulmonary cerebral resuscitation. They did not mention how they provided this photo. If she underwent ophthalmic examination in the first center, what were the ophthalmic findings except fundus appearance? They did not mention any investigation for the etiology of cardiopulmonary cerebral resuscitation need and systemic condition of the patient. Simply did she have systemic hypertension?
In literature, there is no article reporting optic nerve edema caused by Retinal hemorrhage except venous stasis in central retinal vein occlusion. It does not seem that optic nerve edema is caused by such a retinal hemorrhage adjunct to optic nerve. It is reported that optic nerve edema can be seen 20% in Central retinal artery occlusion at initial examination [6]. Therefore, it may be a normal finding in natural course of BRAO. Other factors, which lead to optic nerve edema, optic neuritis, and vasculitis, should be investigated. It may be coincidental condition Valsalva retinopathy and optic neuritis. Therefore, patent foramen ovale-related embolus or carotid artery-related embolus may have played a role in the development of BRAO in this patient.
Umit Yolcu, MD Opthalmology Department Siirt Military hospital
Siirt, Turkey E-mail address: [email protected]
Umit Kaldirim, MD Emergency Medicine Department Siirt Military Hospital, Siirt, Turkey
Abdullah Ilhan, MD Opthalmology Department Erzurum Military Hospital
Erzurum, Turkey
http://dx.doi.org/10.1016/j.ajem.2014.08.011
References
Kim JH, Hyun SY, Kim SM, Chae JB. Valsalva retinopathy and branch retinal artery occlusion after cardiopulmonary cerebral resuscitation. Am J Emerg Med 2014. http://dx.doi.org/10.1016/j.ajem.2014.05.028.
- Sharma S, Brown GC. Retinalarteryocclusion. In: Ryan SJ, editor. Retina. 3rd ed. St. Louis: Mosby; 2001. p. 1350-67.
- Coutu A, Farguette F, Chiambaretta F. Branch retinal artery occlusion secondary to spontaneous carotid artery dissection: case report. J Fr Ophtalmol 2014;37: e79-80 [Epub 2014/05/20. Occlusion d’une branche arterielle retinienne secondaire a une dissection carotidienne spontanee: a propos d’un cas].
- Wilson LA, Warlow CP, Russell RW. Cardiovascular disease in patients with retinal Arterial occlusion. Lancet 1979;1:292-4 [Epub 1979/02/10].
- Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res 2011;30: 359-94 [Epub 2011/05/31].
- Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina 2007;27:276-89 [Epub 2007/04/27].