Prognostic role of copeptin after traumatic brain injury: A systematic review and meta-analysis of observational studies
a b s t r a c t
Purpose: Copeptin, the C-terminal portion of provasopressin, has emerged as a novel prognostic marker in Neurocritical care, such as in Traumatic brain injury . The aim of this study was to quantitatively assess the prognostic significance of initial Plasma copeptin levels in the neurological outcome and mortality after traumatic brain injury.
Materials and methods: Six relevant studies with data from 552 patients were included in this meta-analysis. Results: The plasma copeptin levels were found to be significantly higher in patients who died than in the survivors (standardized mean difference [SMD], 1.80). In the four studies reporting Glasgow Outcome Scale (GOS) data, patients with unfavorable outcomes had significantly higher copeptin levels than those with favorable outcomes (SMD, 1.62). The plasma copeptin level predicted mortality and unfavorable outcomes (AUC, 0.873; AUC, 0.876).
Conclusions: The present meta-analysis suggests that early measurement of plasma copeptin levels can provide better prognostic information about the functional outcome and mortality in patients with TBI.
(C) 2017
Introduction
traumatic brain injury is a leading cause of death and perma- nent disability, especially among young adults [1]. Despite recent ad- vances in the management of TBI in critical care, the mortality and morbidity rates in these patients still high [2]. The age of the patient and initial severity of the injury, as well as radiological imaging based on clinical data, are considered to be independent Prognostic markers of survival after TBI. However, these clinical factors are sometimes insuf- ficient to predict outcomes after TBI in individual patients [3]. New prognostic information, such as that from molecular biomarkers or im- aging tools, is required to enable Early prediction of clinical outcomes in patients with moderate to severe TBI [4]. Early measurement of molec- ular Biological markers could enable more accurate prediction of patient outcomes and provide promising targets for therapeutic intervention.
* Corresponding author at: Department of Preventive Medicine, College of Korean Medicine, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul 130-701, Republic of Korea.
E-mail address: [email protected] (B.-H. Jang).
1 These authors contributed equally to this work.
Over the past several years, biochemical markers of brain injury have been increasingly studied as potential tools for prognostic evaluation [4-6]. Copeptin, the C-terminal portion of provasopressin, is a 39 amino acid-long glycopeptide that is stable at Room temperature. Thus, it can easily be detected using automated assays that can generate results within 30 min[7,8]. Copeptin might also play a role as a delicate surrogate marker for Arginine-vasopressin -release indicating individual stress responses, because AVP is a potent synergistic factor of the hypothalamo-pituitary-adrenal axis. Copeptin is significantly ele- vated in patients with stroke, acute myocardial infarction, Subarachnoid hemorrhages, or TBI, and has also been proposed as a prognostic marker for poor clinical outcome and death in these patients [9-13]. We per- formed a systematic review and meta-analysis of the available evidence in order to quantitatively assess the prognostic value of copeptin in predicting functional outcome and mortality after TBI.
Methods
We used extensive database searching to find studies evaluating the prognostic significance of copeptin after traumatic brain injury, according to the Cochrane review methods [14].
http://dx.doi.org/10.1016/j.ajem.2017.04.038
0735-6757/(C) 2017
Search strategy and data sources
We searched MEDLINE (January 1, 1976 to May 8, 2016), EMBASE
(January 1, 1985 to May 8, 2016), and the Cochrane Library (January
1, 1987 to May 8, 2016) without restrictions based on language or year of publication. The following keywords were searched: copeptin, neurohypophysis hormone, traumatic brain injury, and cerebral hemor- rhage. The search strategies were modified for each database using free text terms and controlled vocabularies. The details of the search strate- gies used are provided in the Supplementary Fig. S1 (online). We also searched the bibliographies of the identified studies and other reviews.
Study selection
The selection of the studies was independently decided by two re- viewers (K.S.C. and Y.S.C.) according to the predefined selection criteria. The reviewers screened the titles and abstracts identified from the elec- tronic searches. The full-length articles for all the relevant studies were obtained and assessed in detail. If studies included duplicate data from a previous study, the study with the most up-to-date results was selected in the analysis. Studies were included in our meta-analysis if they: (1) reported results for patients with traumatic brain injury; (2) measured copeptin levels within 24 h of admission; or (3) assessed functional outcome or mortality during the follow-up period.
Data extraction
The study characteristics and results of the selected studies were ex- tracted by two independent reviewers. Any disagreement unresolved by discussion was put under the review of the other co-authors (W.K. and C.W.A.). The following variables were extracted from studies: first author, year of publication, country, study population, inclusion period, follow-up period, trauma severity according to the Glasgow Coma Scale score, assay method for copeptin detection, functional outcome (Modified Rankin scale [mRS] score or Glasgow Outcome Scale [GOS] score), results of survival analysis, odds ratio (OR) or hazard ratio (HR) or Area under curve values from the receiver operating characteristic curves with 95% confidence interval (CI), and mean copeptin levels with standard deviation (SD); if not available, the medi- an values with the interquartile range (IQR) were used. If the above var- iables were not mentioned in the studies, we asked each corresponding author for the data via email.
Assessment of methodological quality
The methodological quality of the identified studies was assessed independently by B.H.J. and T.H.L., who were blind to the authorship or journal, using the Quality in Prognosis Studies (QUIPS) tool, with values of 0, 1, and 2 considered to be low, unclear, and high, respectively [15]. Studies achieving N 9 points from the sum of each 6-item score were con- sidered to be of high quality [16]. Any unresolved disagreements between the reviewers were resolved through discussion or review from the third author. Publication bias was not assessable in these trials. Tests for funnel plot asymmetry are generally only performed when at least 10 studies are included in the meta-analysis; as our analyses for traumatic brain injury only included 6 studies, tests for asymmetry would be ineffective as they would be unable to differentiate chance from asymmetry.
Statistical analysis
In the main analysis, we investigated the association between the initial copeptin level and unfavorable functional outcome/mortality after traumatic brain injury. We attempted to minimize the within- study reporting bias by using studies reporting both a relative measure of effect (ORs or HRs) and a difference in the means. For pooling the es- timates of the results, we collected the ORs or HRs with 95% CIs. The
pooled HRs or ORs with the 95% CIs were calculated using a random- effects model [17]; the pooled ORs or HRs refer to the copeptin levels on a logarithmic scale with base 10. The strength of association of copeptin levels with the clinical outcome was measured by standard- ized differences in means (difference in means/pooled SD) between the survival and death groups with a random-effects model. Since the copeptin levels were expressed using a wide variety of units in several studies, the results were presented as the standardized mean difference (SMD) and 95% CIs to estimate the size of the effect. The copeptin levels across comparison groups were extracted as mean difference +- SD. When the SD was not available in the included studies, we estimated the variance using the following formulae [15]: SD = standard error (SE) x sqrt(N, sample size), SD = 1.35/IQR. When both univariate and multivariate results were reported, the latter was used in analysis. AUC values were pooled using the mean and SE values, and were weighted using the inverse variance method [18]. Statistical analyses were performed using MedCalc for Windows, version 16.8.4 (MedCalc Software bvba, Ostend, Belgium) [19]. To estimate heterogeneity, we estimated the between-study inconsistency due to true differences be- tween studies (rather than differences due to random error or chance) using the I2 statistic, with values of 25%, 50%, and 75% considered as low, moderate, and high, respectively [20].
We also conducted planned subgroup analyses by stratifying the
total sample based on age (pediatric or adult), injury severity (severe or not), time window of mortality assessment (within 3 months or within 1 year), and the methodological quality of the study (high or low), for the sensitivity analysis. We used RevMan version 5.3 (Cochrane Collaboration, Oxford, UK) for statistical analysis, and results with P b 0.05 were considered statistically significant.
Results
Study selection and characteristics
The process for identifying the eligible studies is shown in Fig. 1. The database search identified 867 articles, of which a total of 698 studies remained after excluding duplicate articles. Of these, 657 irrelevant publications were excluded based on the screening of titles and ab- stracts. A total of 41 potentially relevant studies were fully reviewed with the full text, of which 35 articles were excluded because of the fol- lowing reasons: review articles or abstracts from conferences (n = 8); study design did not fulfill the inclusion criteria (n = 26); or shared an identical population (n = 1). Finally, 552 patients in 6 studies met the inclusion criteria and were included in the meta-analysis [10,21-25]. The main characteristics of the 6 eligible publications are shown in Table 1. All the included studies were observational. Five studies investi- gated traumatic brain injury in adults, while 1 study investigated pediatric brain injury. Overall, the mortality and functional outcome were obtained from all the 5 articles as SMD or AUC values; unfavorable functional out- comes (including mortality) were defined as a GOS score of 1 to 3. Among these studies, 3 studies measured ORs with 95% CIs from multivariate analysis [10,22,23], while 2 studies only compared the mean values of copeptin levels between survivors (favorable outcome) and non-survi- vors (unfavorable outcome) [24,25]. In our meta-analysis, an elevated plasma copeptin level was associated with an increased risk of unfavor- able outcome after TBI (OR, 1.20; 95% CI, 1.05-1.38; I2 = 75%). Despite the lack of a significant association between copeptin level and mortality, the meta-analysis result indicated the tendency of a positive correlation (OR, 1.15; 95% CI, 0.98-1.34; I2 = 95%). The ORs correspond to a 1-unit in- crease in the explanatory variable; for the log-transformed copeptin values, this corresponds to a 10-fold increase. The main outcome was evaluated at 1 month (n = 2), 6 months (n = 2), and 12 months after injury (n = 1). Of the 6 studies, 3 studies controlled for potential confounding factors by adjusting for the effects of known risk factors of poor outcomes after traumatic brain injury, such as severity on the admis-
sion CT scan, GCS score on admission, and others [10,22,23].

Fig. 1. Flow diagram for the identification of relevant studies.
Quality of the included studies
Of the 6 included studies, three studies fulfilled most of the quality criteria [10,22,23] and were deemed to be high quality, while the other two studies did not meet two or more criteria [21, 24,25]. The factors that most affected the quality of the articles were the study confounds and the statistical analysis/presentation. All the included studies specified that the measurement of biomarkers was blind to the clinical data. The details of our quality assessment of the included studies are presented in Supplementary Fig. S2 (online).
Main analysis
Five relevant studies including 481 patients were finally included in our study. All of these studies reported differences in plasma copeptin levels between patients who did not survive and those who did. The copeptin level was found to be significantly higher in patients who died compared to that in survivors, demonstrating a positive association with an overall SMD [(mean level in the death group – mean level in the survival group)/pooled SD] of 1.80 (95% CI, 0.95-2.65; I2 = 92%; P b 0.0001; Fig. 2). However, substantial heterogeneity was present in the data. We evaluated potential sources of heterogeneity through
Characteristics of studies included in the review.
|
Study |
Country |
Sample size |
Inclusion period |
Inclusion criteria |
Blood collection |
Copeptin detection method |
Age, y (range) |
Females,% |
Time of outcome measurement |
Death,% |
Unfavorable outcome, % |
|
Zhang [25] |
China |
102 |
2010-2013 |
Isolated head |
On admission |
ELISA (Phoenix |
40.5 +- 15.3 |
34 (33.3) |
6 months |
29 |
48 (47.1) |
|
trauma, GCS <= 8 |
pharm) |
(18-78) |
(28.4) |
||||||||
|
Lin [23] |
China |
126 |
2010-2012 |
Isolated head |
On admission |
ECLIA |
8 +- 2.7 |
52 (41.2) |
6 months |
21 |
38 (30.2) |
|
trauma, GCS <= 8 |
(B.R.A.H.M.S.) |
(16.7) |
|||||||||
|
Yu [22] |
China |
106 |
2008-2010 |
Isolated head |
On admission |
ELISA (Cusabio) |
45.4 +- 18.4 |
30 (28.3) |
1 year |
31 |
48 (45.3) |
|
trauma, GCS <= 8 |
(29.2) |
||||||||||
|
Dong [10] |
China |
94 |
2007-2010 |
GCS <= 8 |
On admission, |
ELISA (Cusabio) |
42.9 +- 18.6 |
27 (28.7) |
1 month |
26 |
– |
|
days 1, 2, 3, 5, 7 |
(11-80) |
(27.7) |
|||||||||
|
Kleindienst |
Germany |
71 |
2010 |
TBI |
Days 0, 3, 7 |
ECLIA |
53 (18-87) |
14 (19.7) |
– |
– |
– |
|
(B.R.A.H.M.S.) |
|||||||||||
|
Cavus [24] |
Turkey |
53 |
2012 |
On admission, 6h |
ELISA (Phoenix |
26.8 +- 25.2 |
30 (56.5) |
1 month |
11 |
– |
|
|
pharm) |
(1-92) |
(20.7) |
GCS, Glasgow Coma Scale score; ELISA, enzyme-linked immunosorbent assay; ECLIA, electrochemiluminescence immunoassay; y, year; Age were presented as median (IQR) or mean +- SD.
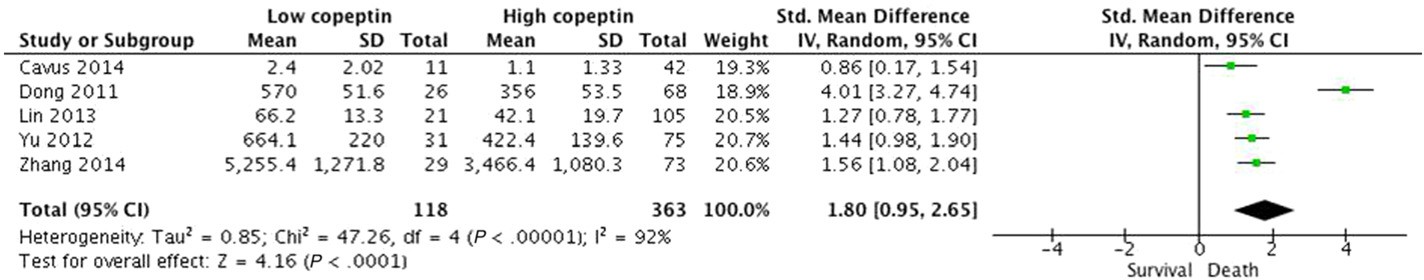
Fig. 2. Standardized mean copeptin values in the death and survival groups and the pooled estimates (random effects model). Five observational studies were included. CI, confidence interval; IV, inverse variance; SD, standard deviation.
additional stratified analyses (Table 2). Despite the significant and con- sistent association between a higher copeptin level and mortality in each subgroup, we observed subgroup differences in the studies based on study design. After excluding a multi-center trial including post- resuscitation patients by Dong et al. [10], higher copeptin level was still related to mortality with no heterogeneity (SMD, 1.35; 95% CI, 1.09-1.60; I2 = 0%). According to this observation, this study may be the key contributor to between-study heterogeneity. However, the asso- ciations did not differ substantially by age, number of female subjects, time of outcome assessment, assay method, or how the mean and SD were obtained (described in published report vs. estimated by recog- nized formulae). In the four studies that evaluated the GOS as neurolog- ical outcome, patients with unfavorable outcomes had significantly higher copeptin concentrations than those with favorable outcomes (SMD, 1.62; 95% CI, 1.19-2.06; I2 = 67%; P b 0.00001; Fig. 3). We ob-
served subgroup differences in studies according to injury severity and how the mean and SD values were obtained (Table 2). Heterogeneity was lower among patients with severe injury or among studies that
described the mean and SD values. However, these associations did not differ substantially by the other characteristics as stated above.
The pooled AUC value of copeptin to predict mortality was signifi- cantly higher than 0.87 (AUC, 0.873; 95% CI, 0.839-0.906; Table 3 and Fig. 4). In addition, among the three studies that evaluated functional outcomes, the pooled AUC value was significantly N 0.87 (AUC, 0.876; 95% CI, 0.838-0.913; Table 4 and Fig. 5). There was no heterogeneity among the pooled AUC values (I2 = 0%; P = 0.4162) of copeptin.
Sensitivity analysis
A sensitivity analysis was performed by the sequentially removing individual studies one at a time and estimating the overall SMD for the remaining studies. The results of the sensitivity analysis suggested that no single study significantly influenced the overall pooled esti- mates, indicating that our results were consistent and statistically reliable.
Stratified meta-analysis of copeptin levels and poor outcome in patients with traumatic brain injury.
|
Characteristics |
Functional outcome |
Mortality |
||||||||
|
N SMD (95% CI) |
P value for heterogeneity |
I2,% |
N SMD (95% CI) |
P value for heterogeneity |
I2,% |
|||||
|
TBI |
||||||||||
|
All |
4 1.62 (1.19, 2.06) |
0.03 |
67 |
5 1.80 (0.95, 2.65) |
b0.00001 |
92 |
||||
|
Injury severity |
||||||||||
|
Mild-moderate |
1 |
0.86 (0.17-1.54) |
– |
– |
1 |
0.86 (0.17, 1.54) |
– |
– |
||
|
Severec |
3 |
1.80 (1.49, 2.11) |
0.25 |
27 |
4 |
2.03 (1.05, 3.02) |
b0.00001 |
93 |
||
|
Age |
||||||||||
|
b18 |
1 |
1.75 (1.31, 2.19) |
– |
– |
1 |
1.27 (0.78, 1.77) |
– |
– |
|
>= 18 Study designa Single center |
3 4 |
1.56 (0.92, 2.20) 1.62 (1.19, 2.06) |
0.01 0.03 |
77 67 |
4 4 |
1.95 (0.84, 3.05) 1.35 (1.09, 1.60) |
b0.00001 0.40 |
93 0 |
|
Multi-center |
0 |
– |
– |
– |
1 |
4.01 (3.27, 4.74) |
– |
– |
|
Number of female |
||||||||
|
b40% |
2 |
1.84 (1.30, 2.38) |
0.10 |
63 |
3 |
2.30 (0.95, 3.66) |
b0.00001 |
95 |
|
>= 40% |
2 |
1.35 (0.47, 2.22) |
0.03 |
78 |
2 |
1.13 (0.73, 1.53) |
0.34 |
0 |
|
Method of estimation of the mean and SDb |
||||||||
|
Described in the study |
3 |
1.80 (1.49, 2.11) |
0.25 |
27 |
3 |
1.43 (1.15, 1.70) |
0.71 |
0 |
|
Estimated by recognized formulas |
1 |
0.86 (0.17-1.54) |
– |
– |
2 |
2.43 (-0.65, 5.51) |
b0.0001 |
97 |
|
Outcome assessment 1 month |
1 |
0.86 (0.17-1.54) |
– |
– |
2 |
2.43 (-0.65, 5.51) |
b0.00001 |
97 |
|
6 months |
2 |
1.92 (1.56, 2.28) |
0.27 |
18 |
2 |
1.42 (1.08, 1.77) |
0.41 |
0 |
|
12 months |
1 |
1.57 (1.13, 2.01) |
– |
– |
1 |
1.44 (0.98, 1.90) |
– |
– |
|
Assay method |
||||||||
|
ELISA |
3 |
1.56 (0.92, 2.20) |
0.01 |
77 |
4 |
1.95 (0.84, 3.05) |
b0.00001 |
93 |
|
ECLIA |
1 |
1.75 (1.31, 2.19) |
– |
– |
1 |
1.27 (0.78, 1.77) |
– |
– |
|
Study quality High |
2 |
1.66 (1.35, 1.97) |
0.57 |
0 |
3 |
2.21 (0.77, 3.65) |
b0.00001 |
95 |
|
Low |
2 |
1.52 (0.28, 2.75) |
0.003 |
88 |
2 |
1.25 (0.57, 1.94) |
0.10 |
63 |
N, the number of studies; SMD, standardized mean difference; 95% CI, 95% confident interval. ELISA, enzyme linked immunosorbent assay; ECLIA, electrochemiluminescence immunoassay.
a Indicate a significant subgroup difference between copeptin level and mortality according to the study design.
b Indicate a significant subgroup difference between copeptin level and unfavorable outcome/mortality according to the study region/method of estimation of the mean and SD.
c Indicate a significant subgroup difference between copeptin level and unfavorable outcome according to the injury severity.
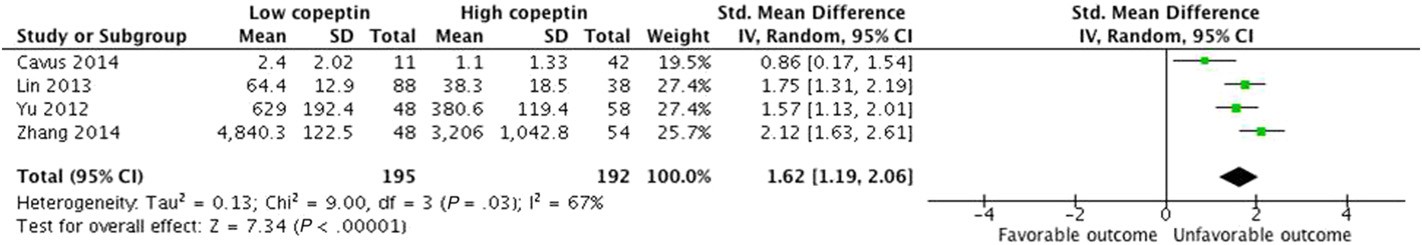
Fig. 3. Standardized mean copeptin values in the unfavorable and favorable outcome groups and the pooled estimates (random effects model). Four observational studies were included. CI, confidence interval; IV, inverse variance; SD, standard deviation.
Discussion
This systematic review and meta-analysis identified that increased copeptin levels were significantly associated with poor neurological outcomes and mortality in patients with TBI. This study has described the SMD for copeptin, thus allowing the clinician to assess the plasma level of copeptin as a practical laboratory variable that may be used to predict clinical outcome. Moreover, a significant association of copeptin with poor clinical outcome after TBI was observed in studies that adjust- ed for important prognostic factors.such as age, sex, and initial injury se- verity. These results suggest the possibility that a higher copeptin level is an independent prognostic factor for mortality and unfavorable func- tional outcome following TBI. This meta-analysis also examined the dis- criminative capacity of plasma copeptin concentrations and found only a moderate discriminatory ability to predict mortality and neurological outcomes in TBI patients. The results show that the prognostic perfor- mance of copeptin measurement would be satisfactory for the manage- ment of patients with TBI. Since the prediction of risk for poor outcomes in patients with acute TBI remains complicated and it mostly depends on underlying conditions or clinical parameters, these findings help in making an informed decision regarding the prognosis.
The prognostic role of copeptin has been reported in various types of
acute illnesses, including hemorrhagic/septic shock, stroke, heart fail- ure, and acute myocardial infarction [8,9,11,26,27,28]; a higher copeptin level has been associated with all of these conditions and also predicts outcomes. Accordingly, copeptin increase as a systemic manifestation might, therefore, lead to an over-estimation of the severity of the cere- bral insult in the acute period after TBI in patients with multiple Organ injuries [29]. All of the 6 studies in this meta-analysis specified enrolling patients with isolated TBI, and five of 6 studies recruited only severely injured patients (GCS score of 8 or less). In addition, the association be- tween the plasma copeptin concentration and clinical outcome was consistent irrespective of the subgroup. Thus, the effect of extracranial sources of copeptin increase is likely to be minimal in this meta-analysis. In contrast to other brain biomarkers, copeptin could directly reflects intracerebral processes and is released into the systemic circulation through its capability of bypassing the blood-brain barrier [30]. In
Table 3 The pooled area under the curve of copeptin to predict mortality in patients with traumat- ic brain injury.
|
Study |
ROC |
Standard |
95% CI |
z |
P |
Weight |
(%) |
|
|
area |
error |
Fixed |
Random |
|||||
|
Zhang, |
0.864 |
0.0362 |
0.793-0.935 |
22.39 |
22.39 |
|||
|
2014 |
||||||||
|
Lin, 2013 |
0.832 |
0.0349 |
0.764-0.900 |
24.09 |
24.09 |
|||
|
Yu, 2012 |
0.909 |
0.0304 |
0.849-0.969 |
31.74 |
31.74 |
|||
|
Dong, 2011 |
0.874 |
0.0367 |
0.802-0.946 |
21.78 |
21.78 |
|||
|
Total (fixed |
0.873 |
0.0171 |
0.839-0.906 |
50.955 |
b0.001 |
100.00 |
100.00 |
|
|
effects) |
||||||||
|
Total |
0.873 |
0.0171 |
0.839-0.906 |
50.955 |
b0.001 |
100.00 |
100.00 |
ROC, receiver operating characteristics; CI, confidence interval.
addition, previous experimental studies identified that vasopressin plays a role in the formation of Brain edema and in ischemic neuronal in- jury, as blocking of vasopressin receptors attenuates brain edema in mice models of ischemic and traumatic injury [31-33].
To the best of our knowledge, this is the first meta-analysis evaluating the prognostic role of copeptin after TBI. The findings of this systematic review and collaborative meta-analysis demonstrated that plasma copeptin level has prognostic value in the assessment of the functional outcome and mortality after TBI. Subgroup analysis identified several im- portant findings. A significant association was observed between the copeptin level and mortality after TBI in the subgroup analysis according to the study design or how the mean and SD values were obtained. Although survival assessment is considered invariable without any intra- or inter-observer differences, this finding should be interpreted with caution since the studies included in this meta-analysis did not describe the direct cause of death or any extracranial complications.
This meta-analysis has several limitations. First, there is a substantial heterogeneity between the studies. Heterogeneity may also result from the different characteristics of the subjects, various treatment modalities, methodological problems, variable measurement assays for copeptin detection, insensitivity of the clinical outcome, and other possible processes contributing to the outcome assessment. However, the stratified analysis based on various study characteristics did not show substantial differences in the results. Thus, we believe that the value of these results should not be underestimated simply because of the overall high heterogeneity, since all the relevant studies consistently revealed a positive association between higher copeptin levels and poor outcome after TBI, and low heterogeneity in the subgroup analysis. Sec- ond, the included studies reported no data related to the serial measure- ment of copeptin. Further studies are needed to evaluate whether serial copeptin measurement further improves the risk stratification in patients with acute TBI. Third, although most studies used multivariate

Fig. 4. The pooled area under the curve values for assessing the mortality (random effects model). Four observational studies were included. ROC, receiver operating characteristics.
Table 4 The pooled area under the curve of copeptin to predict functional outcome in patients with traumatic brain injury.
Author contributions
All the authors contributed to the design of the study. K.S.C. and
Study ROC area
Standard error
95% CI z P Weight (%)
Fixed Random
Y.S.C. performed the database search and screened the studies for their eligibility. B.H.J. and T.H.L. assessed the quality of papers and per-
|
Zhang, 2014 |
0.848 |
0.0378 |
0.774-0.922 |
25.89 |
25.89 |
formed the statistical analysis. K.S.C. and B.H.J. drafted the manuscript. W.K. and C.W.A. moderated the disagreements during data collection |
||
|
Lin, 2013 |
0.863 |
0.0327 |
0.799-0.927 |
34.60 |
34.60 |
and analysis. K.S.C., H.J.Y., and B.H.J. critically revised the manuscript |
||
|
Yu, 2012 |
0.905 |
0.0306 |
0.845-0.965 |
39.51 |
39.51 |
for important intellectual content. All the authors revised the manu- |
||
|
Total (fixed effects) |
0.876 |
0.0192 |
0.838-0.913 |
45.529 |
b0.001 |
100.00 |
100.00 |
script and approved the final version. |
|
Total (random effects) |
0.876 |
0.0192 |
0.838-0.913 |
45.529 |
b0.001 |
100.00 |
100.00 |
Competing financial interests |
ROC, receiver operating characteristics; CI, confidence interval.
analyses to determine an adjusted odds ratio after adjusting for many confounding factors; changes related to factors such as age, sex, and in- jury severity remain a possible alternative explanation for the present study. Forth, publication bias may have been a factor, as negative studies have a lower publication rate and therefore lesser impact, and the lack of published negative studies in the field of biomarkers with regard to prognosis may have affected the results of this meta-analysis [34]. Final- ly, all the original studies were small numbers of observational and most studies from china, therefore, had the limitations related to this type of study that causal relationships cannot be established.
Conclusion
The current systematic review and meta-analysis demonstrates that higher plasma copeptin levels may be a probable prognostic factor for poor functional outcome and mortality in patients with TBI. Based on the current findings, early measurement of plasma copeptin could pro- vide better prognostic information in patients with acute TBI and help in making an informed decision about the therapeutic interventions. Since potential biases and confounds could not be fully excluded in this meta- analysis, future prospective studies should focus on standardizing the assays and identifying Optimal cutoff values of copeptin concentrations. Other patient data such as clinical and laboratory examination results should also be considered to identify other confounds and to improve the prediction of clinical outcome after TBI.

Fig. 5. The pooled area under the curve values for assessing the functional outcome (random effects model). Three observational studies were included. ROC, receiver operating characteristics; CI, confidence interval; HR, hazard ratio; IV, inverse variance; SE, standard error.
The authors have no competing interests. This research did not receive any specific grant from funding agencies in the public, commer- cial, or not-for-profit sectors.
Supplementary data to this article can be found online at http://dx. doi.org/10.1016/j.ajem.2017.04.038.
References
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of Traumatic brain injuries: a global perspective. NeuroRehabilitation 2007;22: 341-53.
- Roozenbeek B, Maas AIR, Menon DK. Changing patterns in the epidemiology of trau- matic brain injury. Nat Rev Neurol 2013;9:231-6.
- MRC CRASH Trial Collaborators. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ 2008;336:425-9.
- Zitnay GA, Zitnay KM, Povlishock JT, Hall ED, Marion DW, Trudel T, et al. Traumatic brain injury Research priorities: the Conemaugh international brain injury sympo- sium. J Neurotrauma 2008;25:1135-52.
- Kovesdi E, Luckl J, Bukovics P, Farkas O, Pal J, Czeiter E, et al. Update on protein bio- markers in traumatic brain injury with emphasis on clinical use in adults and pedi- atrics. Acta Neurochir 2010;152:1-17.
- Kochanek PM, Berger RP, Bayir H, Wagner AK, Jenkins LW, Clark RSB. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr Opin Crit Care 2008;14:135-41.
- Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006;52:112-9.
- Nickel CH, Bingisser R, Morgenthaler NG. The role of copeptin as a diagnostic and Prognostic biomarker for risk stratification in the emergency department. BMC Med 2012;10:7.
- Lipinski MJ, Escarcega RO, D’Ascenzo F, Magalhaes MA, Baker NC, Torguson R, et al. A systematic review and collaborative meta-analysis to determine the incremental value of copeptin for rapid rule-out of acute myocardial infarction. Am J Cardiol 2014;113:1581-91.
- Dong XQ, Huang M, Yang SB, Yu WH, Zhang ZY. Copeptin is associated with mortal- ity in patients with traumatic brain injury. J Trauma 2011;71:1194-8.
- Zhu XD, Chen JS, Zhou F, Liu QC, Chen G, Zhang JM. Detection of copeptin in periph- eral blood of patients with Aneurysmal subarachnoid hemorrhage. Crit Care 2011; 15:R288.
- De Marchis GM, Katan M, Weck A, Fluri F, Foerch C, Findling O, et al. Copeptin adds prognostic information after ischemic stroke results from the CoRisk study. Neurol- ogy 2013;80:1278-86.
- Zweifel C, Katan M, Schuetz P, Siegemund M, Morgenthaler NG, Merlo A, et al. Copeptin is associated with mortality and outcome in patients with acute intracere- bral hemorrhage. BMC Neurol 2010;10:34.
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, ver- sion 5.1.0. Copenhagen: The Nordic Cochrane Centre; 2011.
- Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280-6.
- Choi KS, Kim HJ, Chun HJ, Kim JM, Yi HJ, Cheong JH, et al. Prognostic role of copeptin after stroke: a systematic review and meta-analysis of observational studies. Sci Rep 2015;5:11665.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7: 177-88.
- Zhou XH, Obuchowski NA, DK McClish. Statistical methods in diagnostic medicine. New York: Wiley; 2002.
- MedCalc statistical software version 16.8.4. Ostend, Belgium: MedCalc Software bvba; 2016https://www.medcalc.org.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58.
- Kleindienst A, Brabant G, Morgenthaler NG, Dixit KC, Parsch H, Buchfelder M. Fol- lowing brain trauma, copeptin, a stable peptide derived from the AVP precusor, does not reflect osmoregulation but correlates with injury severity. Acta Neurochir Suppl 2010;106:221-4.
- Yu GF, Huang Q, Dai WM, Jie YQ, Fan XF, Wu A, et al. Prognostic value of copeptin: one-year outcome in patients with traumatic brain injury. Peptides 2012;33:164-9.
- Lin C, Wang N, Shen ZP, Zhao ZY. Plasma copeptin concentration and outcome after pediatric traumatic brain injury. Peptides 2013;42:43-7.
- Cavus UY, Yildirim S, Gurer B, Dibek K, Yilmaz D, Ozturk G, et al. The prognostic value of plasma ?-copeptin levels in patients with isolated traumatic brain injury. Eur J Trauma Emerg Surg 2014;40:373-8.
- Zhang ZY, Zhang LX, Dong XQ, Yu WH, Du Q, Yang DB, et al. Comparison of the per- formances of copeptin and multiple biomarkers in long-term prognosis of severe traumatic brain injury. Peptides 2014;60:13-7.
- Morgenthaler NG. Copeptin: a biomarker of cardiovascular and renal function. Con- gest Heart Fail 2010;16:S37-44.
- Seligman R, Papassotiriou J, Morgenthaler NG, Meisner M, Teixeira PJZ. Copeptin, a novel prognostic biomarker in ventilator-associated pneumonia. Crit Care 2008; 12:R11.
- Katan M, Muller B, Christ-Crain M. Copeptin: a new and promising diagnostic and prognostic marker. Crit Care 2008;12:117.
- Yokobori S, Hosein K, Burks S, Sharma I, Gajavelli S, Bullock R. Biomarkers for the clinical differential diagnosis in traumatic brain injury-a systematic review. CNS Neurosci Ther 2013;19:556-65.
- Urwyler SA, Schuetz P, Fluri F, Morgenthaler NG, Zweifel C, Bergmann A, et al. Prog- nostic value of copeptin: one-year outcome in patients with acute stroke. Stroke 2010;41:1564-7.
- Vakili A, Kataoka H, Plesnila N. Role of arginine vasopressin V1 and V2 receptors for brain damage after transient focal cerebral ischemia. J Cereb Blood Flow Metab 2005;25:1012-9.
- Molnar AH, Varga C, Berko A, Rojik I, Parducz A, Lasclo F, et al. Inhibitory effect of va- sopressin receptor antagonist OPC-31260 on experimental Brain oedema induced by global cerebral ischaemia. Acta Neurochir 2008;150:265-71.
- Trabold R, Krieg S, Scholler K, Plesnila N. Role of vasopressin V(1a) and V2 receptors for the development of secondary brain damage after traumatic brain injury in mice. J Neurotrauma 2008;25:1459-65.
- Garcia-Berrocoso T, Giralt D, Bustamante A, Etgen T, Jensen JK, Sharma JC, et al. B- type Natriuretic peptides and mortality after stroke: a systematic review and meta-analysis. Neurology 2013;81:1976-85.
