Comparison of phenobarbital-adjunct versus benzodiazepine-only approach for alcohol withdrawal syndrome in the ED
a b s t r a c t
Objectives: To compare a phenobarbital-adjunct versus benzodiazepine-only approach for the management of al- cohol withdrawal syndrome in the emergency department (ED) with regard to the need for intensive care unit admission, Severity of symptoms on ED discharge, and complications.
Methods: This was a retrospective cohort study conducted in two academic EDs in the United States. Adult pa- tients seen in the ED with a diagnosis of alcohol withdrawal syndrome were included. Patients were categorized into two groups based on whether phenobarbital was administered in the ED: 1) phenobarbital group (with or without benzodiazepines) or 2) non-phenobarbital group. The primary outcome measure was the need for ICU admission. Secondary outcomes included Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) scores at ED discharge, and complications. Complications were a composite of death, need for intubation, hypotension or vasopressor use, seizures, and hospital acquired pneumonia.
Results: The study cohort included 209 patients (phenobarbital = 97, non-phenobarbital = 112). The mean (standard deviation) age was 49 (12) years and 85% (n = 178) were male. A similar proportion of patients in the phenobarbital (14%, n = 14) and non-phenobarbital (11%, n = 12) groups required ICU admission (p = 0.529). The median CIWA-Ar score on ED discharge was 7 (IQR 4-12) points in the phenobarbital group and 7 (IQR 4-14) points in the non-phenobarbital group (p = 0.752). The occurrence of complications was also similar in the phenobarbital (9%, n = 9) and non-phenobarbital groups (11%, n = 10).
Conclusion: Adjunctive phenobarbital use in the ED for Alcohol withdrawal syndrome did not result in decreased ICU admission, severity of symptoms, or complications.
(C) 2018
Introduction
More than 15 million adults in the United States have Alcohol use disorder, representing approximately 6% of adults [1]. This has placed an additional burden on emergency departments (ED) and the health care system. There are approximately 5 million ED visits annually in the United States due to alcohol consumption [2]. Alcohol use increases gamma-aminobutyric acid (GABAA), an inhibitory neurotransmitter. It also inhibits postsynaptic N methyl D aspartate (NMDA) glutamate-
? Abstract publication/presentation: Western States Conference Annual Meeting, San Diego, CA (May 22nd, 2018).
* Corresponding author at: Pharmacy and Bank Building (A15), Camperdown Campus, University of Sydney, Sydney, New South Wales 2006, Australia.
E-mail address: [email protected] (A.E. Patanwala).
receptor activity. Repeated exposure leads to brain adaptation, and sub- sequent cessation of alcohol consumption can result in Withdrawal symptoms. alcohol withdrawal syndrome is very common in these patients, but some may develop Delirium tremens, which is the most Severe form of AWS. Thus, depending on the outcome of treatment in the ED and severity of AWS, patients may require admission to the in- tensive care unit (ICU), general medical ward, or may be discharged home.
The mainstay of treatment in the ED for AWS has been a symptom triggered approach using benzodiazepines. Phenobarbital is an alterna- tive agent with both GABAA and NMDA receptor effects, which could be advantageous [3]. However, the evidence for the use of phenobarbital for AWS in the ED setting is limited. In one study, patients who received phenobarbital for AWS in the ED were less likely to be admitted to the ICU (8% versus 25%), were less likely to be on a benzodiazepine infusion,
https://doi.org/10.1016/j.ajem.2018.10.007
0735-6757/(C) 2018
1314 S.M. Sullivan et al. / American Journal of Emergency Medicine 37 (2019) 1313-1316
and did not have increased adverse effects [4]. Although these results from a single institution are promising, additional studies are needed to replicate these findings.
The primary objective of this study was to compare a phenobarbital-
adjunct versus benzodiazepine-only approach for the management of AWS in the ED with regard to the need ICU for admission. The secondary objectives were to compare the two approaches with regard to severity of symptoms on ED discharge, and complications.
Methods
Study design and setting
This was a multicenter retrospective cohort study conducted in two affiliated academic EDs in the United States. The total annual ED visits are approximately 100,000 visits per year combined. Both EDs are part of the same department of emergency medicine, with most physicians rotating between practice sites. There was no drug therapy protocol in place for the management of AWS in the ED. Thus, medication selection and dosing was based on provider preference. However, providers had the option to select a symptom triggered benzodiazepine protocol. The Strengthening the Reporting of Observational Studies in Epidemiol- ogy (STROBE) guidelines were followed for all aspects of this study [5]. The Institutional Review Board (IRB) of the University approved the study.
Selection of participants
A list of patients was generated from the electronic medical record for all adult patients (>=18 years) who presented to the ED with a pri- mary diagnosis of AWS based on International Classification of Diseases, 9th or 10th Revision, Clinical Modification [ICD-9-CM, ICD-10-CM] codes (291.81, F10.239). The list was generated for patients presenting to the ED between November 1st, 2013 and September 30th, 2017. The patients on the list were divided into two groups based on whether they received intravenous pHenobarbital in the ED or not. In each group, pa- tients were included in random order using a random number genera- tor until the desired sample was reached. This was done to prevent selection bias as investigators identified patients for inclusion. Each case was manually checked against the medical record to confirm a pri- mary diagnosis of AWS, and medication use for AWS. Patients were ex- cluded from this study if they did not receive phenobarbital or benzodiazepines for the treatment of AWS in the ED.
Data collection and variables
Two investigators (B.D. and S.S.) collected the data and entered it into Research Electronic Data Capture (REDCap) [6]. A third investigator (D.J.) checked a random 10% sample of the data for errors by checking it against each medical record. No systematic errors were seen. The data were then evaluated for errors, discrepancies, and missing values via histograms and summary statistics by a fourth investigators (A.P.). Data collected included demographics, comorbidities, vital signs, labo- ratory parameters, medications, Clinical Institute Withdrawal Assess- ment for Alcohol (CIWA-Ar) scores, complications, as well as length of stay in the ED, ICU and hospital.
Definitions and outcomes
Patients were divided into two groups, based on whether or not they received phenobarbital in the ED. Patients receiving adjunctive pheno- barbital (with or without benzodiazepines) were categorized as the phenobarbital group and those receiving benzodiazepines alone as the non-phenobarbital group. Benzodiazepine use was measured in loraze- pam equivalents using the following ratios: 5 mg diazepam or 2 mg
midazolam or 10 mg chlordiazepoxide = 1 mg lorazepam equivalents [7].
The primary outcome measure was the need for ICU admission. Sec- ondary outcomes included CIWA-Ar scores at ED discharge, and compli- cations. Complications were defined as a composite of death, need for intubation, hypotension or vasopressor use, seizures, or hospital ac- quired pneumonia.
Data analysis
Categorical variables were compared between groups using the Fisher’s exact test. Continuous variables were evaluated for normality visually via the use of histograms. Normally distributed variables were reported as means with standard deviations and compared using the unpaired Student’s t-test. Variables that were not normally distributed were reported as medians with interquartile ranges and compared using the Wilcoxon rank-sum test. Our sample size calculation was based on the effect size from a previous investigation [4]. We assumed the proportion of patients admitted to the ICU would be 25% in the non-phenobarbital group, which would be reduced to 8% with pheno- barbital. Using a power of 80% and a two-tailed alpha of 0.05, we esti- mated that 74 patients would be required in each group (total 148). However, given the uncertainty in our assumptions, we exceeded this target during data collection. All analyses were conducted in STATA 15 (College Station, Texas).
Results
Study cohort
There were 209 patients included in this study. Of these, 97 patients were included in the phenobarbital group and 112 patients were in- cluded in the non-phenobarbital group. The flow from screening to final patient selection is reported in Fig. 1. Overall, the mean (SD) age was 49 (12) years, 85% were male, and 72% were white race. Baseline characteristics were similar between the two groups with a few excep- tions (Table 1). Patients who received phenobarbital were more likely to have a history of alcohol withdrawal seizures, and had a higher total bilirubin (Table 1). Patients in the phenobarbital group received a median first dose of 260 mg (IQR 130-500 mg). These patients also re- ceived a median Cumulative dose of phenobarbital in the ED of 260 mg (IQR 218-650 mg). Most patients in the phenobarbital group also re- ceived benzodiazepines in the ED (81%, 79/97). The median CIWA-Ar score prior to phenobarbital use was 13 (IQR 9-19). AWS symptoms were similar at baseline (Table 1). Vital signs in the ED are reported in Table 2. There was no significant difference in Blood pressure and heart rate at ED entry or discharge between the groups (Table 2).
Main results
A similar proportion of patients in the phenobarbital (14%, n = 14) and non-phenobarbital (11%, n = 12) group required ICU admission (p = 0.529). Also, a similar proportion in both groups were admitted to non-ICU units or discharged home from the ED (Table 3). The median CIWA-Ar score on ED discharge was 7 (IQR 4-12) points in the pheno- barbital group and 7 (IQR 4-14) points in the non-phenobarbital group (p = 0.752). The occurrence of complications was also similar in the phenobarbital (9%, n = 9) and non-phenobarbital groups (11%, n = 10). No patients died in the ED or during hospitalization in the study cohort. ED length of stay and ICU length of stay (in the subset of ICU patients) was similar between groups. However, the phenobarbital group appeared to have a shorter overall hospital length of stay (Table 3). Concurrent medications given to patients such as opioids, hal- operidol, and propofol are reported in Table 4. Overall, other pertinent medication use appeared to be similar between groups.
S.M. Sullivan et al. / American Journal of Emergency Medicine 37 (2019) 1313-1316 1315



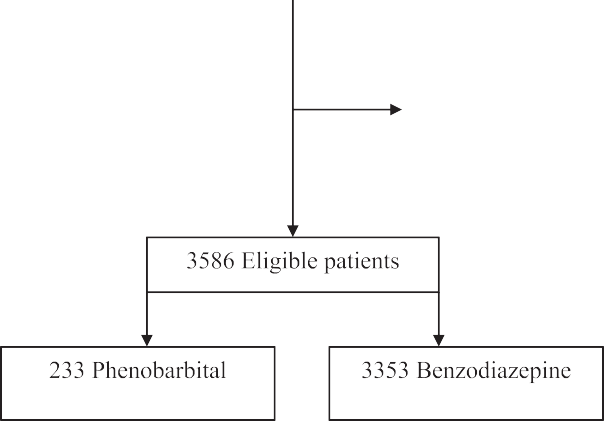


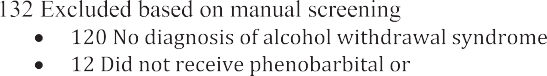

 Fig. 1. Flow diagram of patient selection.
Fig. 1. Flow diagram of patient selection.
Discussion
Baseline characteristics.
|
Variable |
PHEN (N = 97) |
Non-PHEN (N = 112) |
p value |
|
Demographics Age (years), mean (SD) |
48 (12) |
50 (12) |
0.324 |
|
Sex (male), no. (%) |
88 (90) |
90 (80) |
0.050 |
|
Race (white), no. (%) |
67 (69) |
83 (74) |
0.444 |
|
Height (cm), mean (SD) |
173 (8) |
174 (9) |
0.729 |
|
Weight (kg), mean (SD) |
81 (14) |
80 (20) |
0.718 |
|
Comorbidities/history |
The key finding in this study was that the use of phenobarbital for the management of AWS in the ED was not associated with a decrease in ICU admission rates when compared to a benzodiazepine only ap- proach. In addition, we did not find a difference in other outcomes in- cluding AWS symptoms, complications, or overall benzodiazepine consumption. Although hospital length of stay was not a primary out- come for this study, a decreased length of hospital stay (by one day) is an interesting finding. We cannot attribute this to phenobarbital use with certainty given that there were no differences in CIWA-Ar scores at time of discharge from the ED. However, maximum CIWA-Ar score at 24 h was also lower in the phenobarbital group, which is consistent with the potential for a shorter hospital length of stay.
|
Asthma, no. (%) |
7 (7) |
8 (7) |
1.000 |
In a previous trial (n = 102), patients with AWS in the ED were ran- |
|
COPD, no. (%) |
5 (5) |
6 (5) |
1.000 |
domized to receive 10 mg/kg of phenobarbital or placebo. All patients |
were then placed on a symptom guided lorazepam protocol. Patients who received phenobarbital were less likely to be admitted to the ICU
|
Liver disease, no. (%) |
25 (26) |
28 (25) |
1.000 |
|
Alcohol withdrawal seizures, no. (%) |
45 (46) |
33 (30) |
0.015 |
|
Delirium tremens, no. (%) |
9 (9) |
4 (4) |
0.149 |
|
seizure disorder, no. (%) |
15 (15) |
16 (14) |
0.847 |
(8% versus 25%). This suggests that phenobarbital improved symptom |
|
Anti-epileptic use, no. (%) |
10 (10) |
7 (6) |
0.319 |
control of AWS, thereby leading providers to admit these patients to a |
Laboratory values
lower acuity location or discharge them from the ED. This study prompted clinicians in the ED at our institution and many others to
|
AST units/L, median (IQR) |
76 (47-142) |
60 (37-130) |
0.138 |
|
ALT units/L, median (IQR) |
50 (32-84) |
41 (24-72) |
0.066 |
|
Total bilirubin (mg/dL), median (IQR) |
1.1 (0.6-1.5) |
0.8 (0.4-1.3) |
0.008 |
|
Albumin (g/dL) mean, (SD) |
4.1 (0.6) |
4.0 (0.6) |
0.371 |
|
Serum creatinine (mg/dL), median |
0.8 (0.7-1.0) |
0.8 (0.7-1.0) |
0.929 |
|
(IQR) |
|||
|
BUN (mg/dL), median (IQR) |
9 (6-13) |
9 (7-14) |
0.450 |
|
Alcohol level (mg/dL), median (IQR) |
167 |
262 |
0.063 |
|
(38-280) |
(78-347) |
Vital signs in the emergency department.
Variable PHEN
(N = 97)
Non-PHEN (N = 112)
p value
Initial CIWA-Ar score components Prior to first dose
PHEN = phenobarbital group; Non-PHEN = non-phenobarbital group; CIWA-Ar = Clin- ical Institute Withdrawal Assessment for Alcohol.
Heart rate (beats/min), mean (SD) 92 (15) 91 (17) 0.559
PHEN = phenobarbital group; Non-PHEN = non-phenobarbital group.
|
Tremor, median (IQR) |
3 (1-5) |
3 (2-4) |
0.79 |
Systolic blood pressure (mm Hg), mean (SD) |
144 (19) |
142 (22) |
0.470 |
|
Sweats, median (IQR) |
1 (0-2) |
1 (0-2) |
0.50 |
Diastolic blood pressure (mm Hg), mean (SD) |
88 (14) |
86 (16) |
0.214 |
|
Agitation, median (IQR) |
1 (0-2) |
0 (0-2) |
0.93 |
Heart rate (beats/min), mean (SD) |
105 (18) |
102 (18) |
0.249 |
|
Anxiety, median (IQR) |
2 (1-3) |
2 (1-3) |
0.74 At emergency department discharge |
||||
|
Orientation, median (IQR) |
0 (0-1) |
0 (0-1) |
0.64 |
Systolic blood pressure (mm Hg), mean (SD) |
137 (17) |
137 (20) |
0.980 |
|
Hallucinations, median (IQR) |
0 (0-2) |
0 (0-2) |
0.86 |
Diastolic blood pressure (mm Hg), mean (SD) |
84 (12) |
83 (15) |
0.709 |
1316 S.M. Sullivan et al. / American Journal of Emergency Medicine 37 (2019) 1313-1316
Table 3
Outcomes.
Table 4
Medication use.
|
Variable |
PHEN (N = 97) |
Non-PHEN (N = 112) |
p value |
Variable |
PHEN (N = 97) |
Non-PHEN (N = 112) |
p value |
|
|
Disposition ICU, no. (%) |
14 (14) |
12 (10) |
0.529 |
Benzodiazepine use ED use, median (IQR)a |
4 (2-10) |
5 (3-11) |
0.162 |
|
|
Non-ICU, no. (%) |
41 (42) |
60 (54) |
0.127 |
Time to first ED dose (hours), median (IQR) |
1.6 |
1.1 |
0.261 |
|
|
Discharge from ED, no. (%) Complications |
42 (43) |
40 (36) |
0.320 |
PRN use during admission, median (IQR)a |
(0.5-4.7) 10 (2-24) |
(0.5-3.0) 12 (4-38) |
0.128 |
|
|
Required intubation, no. (%) |
6 (6) |
7 (6) |
1.000 |
Scheduled use during admission, median |
7 (3-12) |
10 (4-24) |
0.136 |
|
|
Hospital acquired pneumonia, no. (%) |
4 (4) |
3 (3) |
0.707 |
(IQR)a |
||||
|
Hypotension or vasopressor use, no. (%) |
3 (3) |
0 (0) |
0.098 |
Total use during admission, median (IQR)a |
14 (8-33) |
22 (9-58) |
0.160 |
|
|
Seizure, no. (%) |
3 (3) |
5 (4) |
0.727 |
Benzodiazepine infusion given, no. (%) |
0 (0) |
2 (2) |
0.500 |
|
|
Length of stay |
Other medications |
|||||||
|
ED length of stay (hours), median (IQR) |
9 (6-14) |
9 (6-14) |
0.647 |
Opioids given, no. (%) |
10 (10) |
18 (16) |
0.309 |
|
|
ICU length of stay (days), median (IQR) |
2 (1-3) |
2 (1-6) |
0.745 |
Haloperidol given, no. (%) |
4 (4) |
9 (8) |
0.268 |
|
|
Hospital length of stay (days), median |
3 (2-5) |
4 (2-6) |
0.048 |
Propofol given, no. (%) |
5 (5) |
3 (3) |
0.476 |
|
|
(IQR) ED = emergency department; PRN = as needed; PHEN = phenobarbital group; Non- CIWA-Ar scores PHEN = non-phenobarbital group. |
||||||||
a Expressed in mg lorazepam equivalents.
|
Score on ED discharge, median (IQR) |
7 (4-12) |
7 (4-14) |
0.752 |
|
Maximum score in ED, median (IQR) |
15 (11-22) |
14 (9-20) |
0.454 |
|
Maximum score at 24 h, median (IQR) |
13 (9-18) |
16 |
0.045 |
|
(11-21) |
|||
|
Maximum score at 48 h, median (IQR) |
12 (5-16) |
12 (8-17) |
0.302 |
PHEN = phenobarbital group; Non-PHEN = non-phenobarbital group; ED = emergency department; ICU = intensive care unit; CIWA-Ar = Clinical Institute Withdrawal Assess- ment for Alcohol.
consider the adjunctive use of phenobarbital in this setting. However, we were unable to replicate these findings. One explanation for this dif- ference is that a lower dose of phenobarbital was used in our study, which did not provide the same benefit. For example, the median dose in our study was 260 mg, whereas the dose in the trial mentioned above was 10 mg/kg (i.e. 700 mg for a 70 kg adult). Anecdotally, we are aware that physicians and pharmacists were not comfortable with the higher dose used in the study mentioned above due to the potential for central nervous system and respiratory depression. According to FDA labeling, the use of phenobarbital for AWS would be considered off-label [8]. Dosing is extrapolated based on other indications. The larg- est intravenous dose listed by the FDA is up to 320 mg for bedtime hyp- nosis or acute convulsions [8].
Phenobarbital has some theoretical benefits over benzodiazepines alone from a mechanistic perspective. Chronic alcohol use leads to down regulation of GABAA receptors and up regulation of NMDA recep- tors. Abrupt withdrawal of alcohol use leads to greater NMDA receptor mediated excitatory activity, which may be inhibited more effectively with phenobarbital rather than benzodiazepines [3]. However, the role of phenobarbital for AWS remains unclear because there is an over- all paucity of studies regarding its use for AWS. In the ICU setting, proto- cols that utilized phenobarbital for Refractory cases as part of an escalation strategy reduced ICU length of stay, duration of mechanical ventilation, and decreased benzodiazepine use [9,10]. However, these were small retrospective studies and it is difficult to extrapolate the findings to the ED setting.
The study has some important limitations. First, given that this was a
retrospective study, we were dependent on accuracy of documentation in the electronic medical record. However, the primary outcome was ICU admission and disposition status is automated rather than requiring manual documentation, thus an error within the medical record is not expected to occur. Second, although baseline characteristics were simi- lar between groups with regard to most variables, patients in the phe- nobarbital group had a higher total bilirubin level and were more likely to have a history of alcohol withdrawal seizures. Thus, we cannot exclude the possibility of selection bias. It is possible that providers were more likely to use phenobarbital in patients that they expected to
experience more serious AWS. Third, dosing of phenobarbital was based upon provider discretion and therefore not standardized. The doses used in our study were lower than a previous investigation show- ing an outcome benefit. Hence we cannot extrapolate the results to higher doses of phenobarbital. Future studies should focus on elucidat- ing which dosing strategy for phenobarbital is most effective while still safe for patients experiencing alcohol withdrawal.
Conclusions
At the doses used in this investigation, adjunctive phenobarbital in the management of AWS in the ED did not result in reduced ICU admis- sion rates or severity of AWS symptoms at time of ED transfer or dis- charge. However, it did not increase complications.
Conflicts of interest and source of funding
None.
References
- Alcohol facts and statistics. National Institute of Alcohol Abuse and Alcoholism Avialable from: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol- consumption/alcohol-facts-and-statistics, Accessed date: 1 August 2018.
- White AM, Slater ME, Ng G, Hingson R, Breslow R. Trends in alcohol-related emer- gency department visits in the United States: results from the nationwide emer- gency department sample, 2006 to 2014. Alcohol Clin Exp Res 2018;42:352-9.
- Schmidt KJ, Doshi MR, Holzhausen JM, Natavio A, Cadiz M, Winegardner JE. Treat- ment of severe alcohol withdrawal. Ann Pharmacother 2016;50:389-401.
- Rosenson J, Clements C, Simon B, Vieaux J, Graffman S, Vahidnia F, et al. Phenobarbi- tal for acute alcohol withdrawal: a prospective randomized double-blind placebo- controlled study. J Emerg Med 2013;44:592-8 [e2].
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61:344-9.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42: 377-81.
- Bezchlibnyk-Butler KZ, Jeffries JJ, editors. Clinical handbook of psychotropic drugs. 4th revised ed. Clarke Institute of Psychiatry, Toronto: Hogrefe & Huber; 1991.
- Phenobarbital sodium injection [packing insert]. Eatontown, NJ 07724 USA: West- Ward Pharmaceuticals; 2011 (Revised June 2011) , Accessed date: 10 August 2018.
- Duby JJ, Berry AJ, Ghayyem P, Wilson MD, Cocanour CS. Alcohol withdrawal syn- drome in critically ill patients: protocolized versus nonprotocolizED management. J Trauma Acute Care Surg 2014;77:938-43.
- Gold JA, Rimal B, Nolan A, Nelson LS. A strategy of escalating doses of benzodiaze- pines and phenobarbital administration reduces the need for mechanical ventilation in delirium tremens. Crit Care Med 2007;35:724-30.
