High risk clinical characteristics for pyogenic spinal infection in acute neck or back pain: Prospective cohort study
a b s t r a c t
Objective: To identify clinical characteristics associated with pyogenic spinal infection among adults presenting to a community emergency department (ED) with neck or back pain. A secondary objective was to describe the fre- quency of these characteristics among patients with spinal Epidural abscess (SEA).
Methods: We conducted a prospective cohort study in a community ED enrolling adults with neck or back pain in whom the ED provider had Clinical concern for pyogenic spinal infection. Study Phase 1 (Jan 2004-Mar 2010) in- cluded patients with and without pyogenic spinal infection. Phase 2 (Apr 2010-Aug 2018) included only patients with pyogenic spinal infection. We performed univariate and multivariate analyses for association of clinical characteristics with pyogenic spinal infection.
Results: We enrolled 232 and analyzed 223 patients, 89 of whom had pyogenic spinal infection. The median age was 55 years and 102 patients (45.7%) were male. The clinical characteristics associated with pyogenic spinal in- fection on multivariate analysis of study phase 1 included recent soft tissue infection or bacteremia (OR 13.5, 95% CI 3.6 to 50.7), male sex (OR 5.0, 95% CI 2.5 to 10.0), and fever in the ED or prior to arrival (OR 2.8, 95% CI 1.3 to 6.0). Among patients with SEA (n = 61), 49 (80.3%) had at least one historical risk factor, 12 (19.7%) had fever in the ED, and 8 (13.1%) had a history of intravenous drug use.
Conclusion: Male sex, fever, and recent soft tissue infection or bacteremia were associated with pyogenic spinal infection in this prospective ED cohort.
(C) 2019
Pyogenic spinal infections include Spinal epidural abscess (SEA), ver- tebral osteomyelitis, septic facet joint, paravertebral abscess, and paraspinal abscess [1-3]. The incidence of spinal infections is increasing due to an aging population, greater use of spinal instrumentation, and larger burden of comorbidities [4-6]. Prospective data from the emer- gency department (ED) setting are limited to inform expert recommen- dations for ED diagnosis of pyogenic spinal infections [7-10]. The majority of studies include patients from primary care, intensive care unit, and other settings making it difficult to describe ED patients specif- ically. The largest ED specific study examined 86 cases of SEA at a single
* Corresponding author at: San Antonio Military Medical Center, Department of Emergency Medicine, 3551 Roger Brooke Dr, Fort Sam Houston, TX 78234, United States of America.
E-mail addresses: [email protected] (W.T. Davis), [email protected] (M.D. April).
academic ED [11]. The generalizability of these data to other ED settings remains unclear as the 60% prevalence of prior Intravenous drug use contrasts with the 8.8% prevalence of IVDU reported in a review of 915 SEA cases [11,12].
Importance
Pyogenic spinal infections pose a diagnostic challenge to emergency providers. Previous authors have questioned the validity of the fre- quently cited historical risk factors for pyogenic spinal infection [9,13]. Despite this, current recommendations for evaluation of spinal infection in the ED recommend a risk factor and Neurologic assessment coupled with inflammatory biomarkers to identify patients in need of urgent or emergent Spinal magnetic resonance imaging [7,11]. Delay in diagnosis of SEA is associated with increased risk of permanent neuro- logic deficits [14]. Yet, spinal MRI, the diagnostic imaging modality of choice, is expensive and time consuming [4,9,15]. Additional data clari- fying the characteristics of patients presenting with these deadly and elusive diagnoses may inform imaging protocols to help ED providers avoid missing this diagnosis and better target those patients requiring
https://doi.org/10.1016/j.ajem.2019.05.025
0735-6757/(C) 2019
urgent MRI [16,17]. Such data will ideally encompass patients with any pyogenic spinal infection and not just SEA given the risk of permanent neurologic deficits (3-32%) from pyogenic vertebral osteomyelitis [18- 22]. Data describing patients with pyogenic spinal infection in commu- nity ED settings are lacking.
Goals of this investigation
We identified high risk clinical characteristics for pyogenic spinal in- fection among adults presenting to a community ED with neck or back pain. We described the frequency of those characteristics among adults with SEA and non-SEA pyogenic spinal infection, which included any combination of vertebral osteomyelitis, septic facet joint, paravertebral abscess, and paraspinal abscess.
- Materials and methods
- Study design and setting
This was a single-center, prospective, observational study at a com- munity hospital located in the metropolitan area of a major southwest- ern U.S. city. The annual ED census was approximately 50,000 patients during the study period. The hospital institutional review board ap- proved the study protocol. We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) state- ment in our research design, reporting, and analysis [23].
Selection of participants
We enrolled a convenience sample of adult patients presenting to the ED from January 2004 to August 2018. Adults (>=17 years old) pre- senting to the ED with neck or back pain in whom the ED provider was clinically concerned for pyogenic spinal infection were potentially eligible for study inclusion. Possible prompts for patient enrollment in- cluded fever, recent spinal procedure, multiple recent visits for unex- plained spinal pain, the presence of published risk factors for spinal infection, new neurologic deficits or clinical gestalt suggesting spinal in- fection. We excluded patients diagnosed with fungal or tuberculous spi- nal infection to allow comparison to other similar studies [2,14]. We excluded patients who underwent spinal surgery b5 days prior to ED presentation as inflammatory markers remain elevated during this pe- riod whether or not infection is present [24-26].
ED providers received education regarding the study including in- clusion and exclusion criteria and patient recruitment procedures through quarterly group communications. During study phase 1 (Janu- ary 2004-March 2010), ED providers contacted the principal investiga- tor (PI) whenever ordering a C-reactive protein (CRP) and/or spinal MRI for the express purpose of evaluation for spinal infection. Thus, study phase 1 entailed enrollment of patients with and without spinal infec- tions. During study phase 2 (April 2010-August 2018), ED providers followed the same protocol for contacting the PI, but we enrolled only patients diagnosed with spinal infection in the ED or during the index hospitalization. PI availability determined enrollment times. The PI per- sonally screened all patients for enrollment. All enrolled patients re- ceived routine care and provided verbal consent as per the wavier for documentation of informed consent.
Methods of measurement
The PI personally completed all data collection by interviewing each patient, performing a focused physical examination, and prospectively abstracting data from the patient’s medical record. Historical data col- lected included demographics, history of measured fever (>=38 ?C), and pain characteristics. Additional history included the presence of histor- ical risk factors as identified by a comprehensive review of the literature describing SEA: diabetes, IVDU, immunosuppression, dialysis, active
malignancy, recent soft tissue infection or bacteremia (defined as posi- tive blood culture or hospitalization with infection concerning for bac- teremia within 2 weeks of presentation), indwelling vascular catheter, presence of spinal implant, cirrhosis, vertebral fracture within 2 weeks of presentation, and spinal surgery or injection (including epidural ste- roid injection or epidural catheter placement) within 3 months of pre- sentation [12,27-30]. Data collected by physical examination included an examination of each patient’s back. Neurologic examination included evaluation of sensation of the perineum and strength, sensation, and re- flexes of all extremities. We recorded whether back pain made a patient unable to transfer from a supine position to an upright sitting position without assistance for back examination. Data recorded from the med- ical record at the time of the initial ED visit included vital signs at pre- sentation. Data prospectively collected from the medical record once available following the initial ED visit included operative findings, dis- charge diagnosis, and the results of any cultures of blood, needle aspira- tion, or operative wounds. Additional data collected included formal written interpretations of all MRI studies by neuroradiologists. The PI recorded all collected data on a hard copy instrument.
Outcome measures
Pyogenic spinal infection diagnoses included SEA, vertebral osteo- myelitis, paraspinal abscess, paravertebral abscess or septic facet joint [2]. Providers could reach these diagnoses in one of three ways:
1) MRI evidence of spinal infection as documented by a neuroradiolo- gist, 2) surgical findings consistent with spinal infection as documented in an operative report, or 3) positive culture by Needle aspiration of the spinal infection. Radiologists were provided with the usual clinical in- formation at our institution and were blinded to the data collected spe- cifically for the study. Two investigators independently reviewed all MRI reports for location and diagnosis of spinal infection.
We categorized patients as negative for pyogenic spinal infection if an MRI was negative for pyogenic spinal infection; spinal symptoms re- solved without antibiotics on telephone follow up; or a follow up period of at least six months in the medical record did not reveal a diagnosis of spinal infection, new neurologic deficits, or death due to spinal infec- tion. This extended follow up period was chosen as many patients have multiple months of symptoms prior to diagnosis of spinal infection [3,31]. All patients did not receive a spinal MRI due to the observational nature of the study. The PI placed a telephone call between 14 and 28 days after discharge to patients without a diagnosis of spinal infec- tion. We reviewed available medical records for a period of up to 3 years following the index presentation for all patients without a diag- nosis of spinal infection. The PI queried these sources for evidence of neurologic deficits or diagnosis of spinal infection. We reviewed death records looking for any patient who was lost to follow up through the above sources. Among patients not receiving a spinal MRI, we excluded patients who did not confirm resolution of spinal symptoms at the fol- low up telephone call and had less than six months of follow up data available in the medical record.
Data analysis
Two investigators double entered all hard-copy data collection forms into an Excel database (version 14; Microsoft, Redmond, WA). We exported all data into SPSS (version 22; IBM, Armonk, NY) for statis- tical analysis. We reported all collected data for each patient regardless of whether data were missing for some study variables.
We performed descriptive statistics for clinical characteristics of all patients in the cohort. For patients enrolled in phase 1 of the study prior to March 2010, we performed a univariate analysis to compare clinical characteristics among patients with and without pyogenic spi- nal infection. Next, we constructed a multivariate binary logistic regres- sion model with pyogenic spinal infection as the dependent variable. We included history components and Physical exam findings as co-
variates. We performed descriptive statistics for the clinical characteris- tics in all enrolled patients with SEA versus non-SEA pyogenic spinal in- fections to allow comparison of our cohort to previous studies focusing solely on SEA. Non-SEA pyogenic spinal infections included patients with any combination of vertebral osteomyelitis, septic facet joint, paravertebral abscess, or paraspinal abscess [2]. We compared data among patients with pyogenic spinal infection enrolled before versus after March 31, 2010 to analyze possible effects from the change in study protocol enrollment process (see Appendix).
- Results
- Characteristics of study subjects
We approached 261 patients and enrolled 232 patients during 2004-2018 (Fig. 1). We excluded nine patients: three patients were lost to follow up after not receiving an MRI to exclude spinal infection, two patients were diagnosed with fungal spinal infections, and three patients enrolled in study phase 2 did not meet criteria for spinal infec- tion on case adjudication. Another excluded patient died two weeks fol- lowing ED presentation with a clinical diagnosis of pneumonia but never received a spinal MRI or autopsy to definitively rule out spinal in- fection. In study phase 1, we analyzed 174 patients, of whom 40 had spi- nal infection. In study phase 2, we analyzed 49 patients, all of whom had spinal infections. The median age was 55 years (interquartile range [IQR] 41 to 66 years). Approximately half of the patients were male (45.7%).
Main results
Final diagnoses included nonspecific back pain (40.8%), pyogenic spinal infection (39.9%), other emergent spinal conditions (11.7%) such as epidural hematoma or metastatic cancer, and non-spine diagno- ses (7.6%) such as pyelonephritis or pneumonia (Table 1). SEA was pres- ent in the majority (68.5%) of pyogenic spinal infections. The majority of patients were evaluated with spinal MRI (84.3%) and were admitted to the hospital (70.9%).
Table 1
Final diagnosis of 223 analyzed patients.
Final diagnosis No. of patients (%)
Pyogenic spinal infection 89 (39.9)
Spinal epidural abscess 61 (27.4)
Vertebral osteomyelitis 54 (24.2)
Septic facet joint 15 (6.7)
Paraspinous abscess 37 (16.6)
Paravertebral abscess 11 (4.9)
Metastatic cancer 7 (3.1)
Epidural hematoma 9 (4.0)
Central disc herniation 8 (3.6)
Meningitis or myelitis 2 (0.9)
Nonspecific back pain 91 (40.8)
Non-spine diagnosis 17 (7.6)
The clinical characteristics most strongly associated with pyogenic spinal infection on univariate analysis of patients enrolled in study phase 1 (Table 2) were recent soft tissue infection or bacteremia (odds ratio [OR] 26.2, 95% confidence interval [CI] 7.1 to 97.2), male sex (OR 7.1, 95% CI 3.2 to 15.8), and having at least one historical risk factor (OR 7.1, 95% CI 2.1 to 24.3). Odds ratios for indwelling vascular catheter and IVDU history could not be calculated as no patient in the non-infection group had either risk factor. Notable characteristics which were not significantly associated with pyogenic spinal infection included recent spinal injection, radicular pain (OR 0.9) and midline spi- nal tenderness (OR 0.6).
The presence of new neurologic deficits had weak associations with pyogenic spinal infection (Table 2). Among patients with emergent spi- nal diagnoses other than pyogenic spinal infection (n = 26), the major- ity (57.7%) had a neurologic deficit on presentation to the ED. When excluding these 26 patients from the non-infection group, neurologic deficits had the following associations with pyogenic spinal infection: urinary incontinence (OR 5.2, 95% CI 1.6 to 16.9), Extremity weakness
(OR 3.6, 95% CI 1.3 to 10.2), extremity numbness (OR 3.6, 95% CI 1.0 to
12.7), abnormal reflex exam (OR 15.3, 95% CI 1.7 to 135.3), and any new neurologic deficit (OR 4.4, 95% CI 1.8 to 10.4).
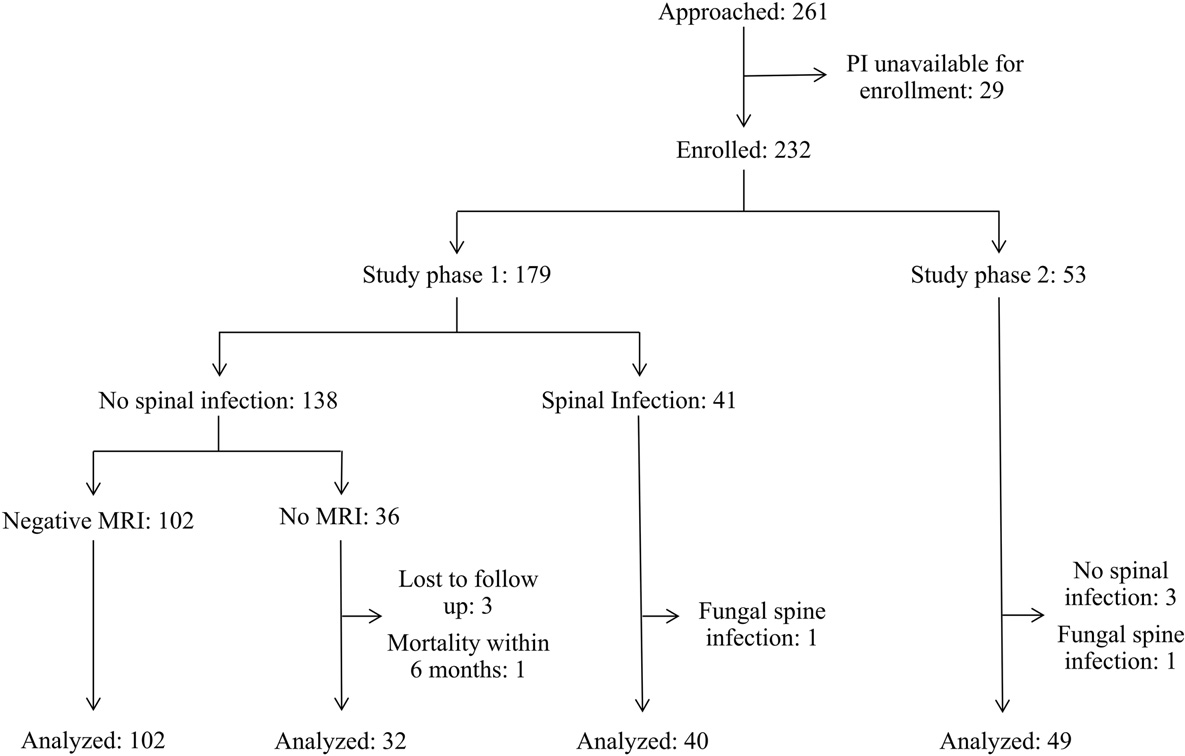
Fig. 1. Patient flow diagram. Enrollment periods were January 2004-March 2010 for study phase 1 and April 2010-2018 for study phase 2.
Univariate analysis of clinical characteristics association with pyogenic spinal infection among patients enrolled in study phase 1.
|
Pyogenic spinal infection (n = 40) |
No spinal infection (n = 134) |
Odds ratio (95% CI) |
|
|
Median age (IQR), y |
51.5 (41.8 to 59.3) |
55.5 (38 to 69.8) |
|
|
Male sex |
30 (75) |
40 (29.9) |
7.1 (3.2 to 15.8) |
|
Historical risk factors |
|||
|
>=1 risk factor present |
37 (92.5) |
85 (63.4) |
7.1 (2.1 to 24.3) |
|
IVDU history |
3 (7.5) |
0 (0) |
- |
|
Dialysis |
3 (7.5) |
4 (3.0) |
2.6 (0.6 to 12.3) |
|
Indwelling vascular catheter |
4 (10) |
0 (0) |
- |
|
Recent SSTI or bacteremiaa |
15 (37.5) |
3 (2.2) |
26.2 (7.1 to 97.2) |
|
Immunosuppression |
2 (5) |
4 (3.0) |
1.7 (0.3 to 9.7) |
|
Active malignancy |
2 (5) |
4 (3.0) |
1.7 (0.3 to 9.7) |
|
Diabetes |
17 (42.5) |
39 (29.1) |
1.8 (0.9 to 3.7) |
|
Cirrhosis |
3 (7.5) |
0 (0) |
- |
|
Spinal implant present |
0 (0) |
7 (5.2) |
- |
|
Recent vertebral fractureb |
0 (0) |
5 (3.7) |
- |
|
Recent spinal surgeryb |
14 (35) |
24 (17.9) |
2.5 (1.1 to 5.4) |
|
Recent spinal injectionb |
0 (0) |
21 (15.7) |
- |
|
Reported symptoms |
|||
|
Radicular pain |
17 (42.5) |
59 (44) |
0.9 (0.5 to 1.9) |
|
Urinary incontinenced |
8 (20) |
8 (6) |
3.9 (1.4 to 11.3) |
|
History of measured feverc |
19 (47.5) |
21 (15.7) |
4.9 (2.2 to 10.6) |
|
Physical exam findings Fever in EDc |
14 (35) |
23 (17.2) |
2.6 (1.2 to 5.7) |
|
Midline spinal tenderness |
11 (27.5) |
50 (37.3) |
0.6 (0.3 to 1.4) |
|
Inability to sit independently |
15 (37.5) |
30 (22.4) |
2.1 (1.0 to 4.4) |
|
Extremity weaknessd |
9 (22.5) |
21 (15.7) |
1.6 (0.7 to 3.8) |
|
Extremity numbnessd |
6 (15) |
14 (10.4) |
1.5 (0.5 to 4.2) |
|
Abnormal reflex examd |
5 (12.5) |
5 (3.7) |
3.7 (1.0 to 13.5) |
|
Any new neurologic deficite |
15 (37.5) |
28 (20.9) |
2.3 (1.1 to 4.9) |
Data are presented as No. (%) unless otherwise indicated.
a Defined as a skin and soft tissue infection (SSTI), positive blood culture, or infection requiring hospitalization within 2 weeks of presentation.
b Recent was defined as within 2 weeks for vertebral fracture and within 3 months for recent spinal surgery or injection.
c Temperature >= 38 ?C.
d Developed within the last two weeks per assessment by principal investigator.
e Neurologic deficits included motor weakness, Urinary retention, numbness, or abnormal reflexes.
Table 3 displays the output of the multivariate logistic regression model for the presence of pyogenic spinal infection in patients enrolled during study phase 1 (2004-2010). The predictor variables associated with pyogenic spinal infection included recent soft tissue infection or bacteremia (OR 13.5, 95% CI 3.6 to 50.7), male sex (OR 5.0, 95% CI 2.5 to 10.0), any fever in the ED or measured prior to arrival (OR 2.8, 95% CI 1.3 to 6.0), and inability due to pain to transfer independently from supine to upright sitting position for back examination (OR 2.2, 95% CI 1.0 to 4.5).
The clinical characteristics were similar between patients with SEA (n = 61) and non-SEA (n = 28) pyogenic spinal infections enrolled from 2004 to 2018 in study phases 1 and 2 (Table 4). The most common historical risk factors among patients with SEA were diabetes mellitus
Table 4
Clinical characteristics of all patients enrolled from 2004 to 2018 with pyogenic spinal in- fection stratified by the presence of spinal epidural abscess (SEA).
|
Spinal epidural abscess, n = |
Non-SEA spinal infection, n = 28 |
|
|
61 (%) |
(%) |
|
|
Median age (IQR), y |
55 (46 to 61) |
57 (48.5 to 68.3) |
|
Male sex Historical risk factors |
44 (72.1) |
18 (64.3) |
>=1 risk factor present 49 (80.3) 25 (89.3)
IVDU history 8 (13.1) 0 (0)
Dialysis 2 (3.3) 3 (10.7)
(37.7%) or a recent soft tissue infection or bacteremia (36.1%). Among
Indwelling vascular
catheter
8 (13.1) 3 (10.7)
Multivariable analysis of association between clinical characteristics and pyogenic spinal infection among 174 patients enrolled from 2004 to 2010 in study phase 1.
|
Variable Ad |
justed odds ratio 95% CI |
P value |
Spinal implant present Recent vertebral fracture |
1 (1.6) 0 (0) |
1 (3.6) 0 (0) |
|
|
Age |
1.0 |
1.0 to 1.0 |
0.857 |
Recent spinal surgery |
10 (16.4) |
10 (35.7) |
|
Male sex |
5.0 |
2.5 to 10.0 |
b0.001 |
Recent spinal injection |
5 (8.2) |
4 (14.3) |
|
Dialysis Recent SSTI or bacteremia |
0.6 13.5 |
0.1 to 4.4 3.6 to 50.7 |
0.601 b0.001 |
Reported symptoms Radicular pain |
33 (54.1) |
12 (42.9) |
|
Immunosuppression |
1.3 |
0.2 to 7.8 |
0.756 |
Urinary incontinence |
12 (19.7) |
3 (10.7) |
|
Cancer |
0.3 |
0 to 2.2 |
0.226 |
History of measured fever |
14 (23.0) |
14 (50.0) |
|
Diabetes |
1.9 |
0.9 to 3.9 |
0.078 |
Physical exam findings |
||
|
Recent spinal surgery |
1.8 |
0.8 to 4.1 |
0.174 |
Fever in ED |
12 (19.7) |
10 (35.7) |
|
Radicular pain |
1.7 |
0.8 to 3.4 |
0.165 |
Midline spinal tenderness |
19 (31.1) |
11 (39.3) |
|
Any fevera |
2.8 |
1.3 to 6.0 |
0.006 |
Inability to sit |
30 (49.2) |
8 (28.6) |
|
Midline spinal tenderness |
1.1 |
0.5 to 2.2 |
0.828 |
independently |
||
|
Inability to sit independently |
2.2 |
1.0 to 4.5 |
0.040 |
Extremity weakness |
11 (18.0) |
6 (21.4) |
|
Any new neurologic deficit |
1.7 |
0.8 to 3.7 |
0.191 |
Extremity numbness |
6 (9.8) |
4 (14.3) |
|
Constant |
0.1 |
Abnormal reflex exam |
8 (13.1) |
1 (3.6) |
||
Recent SSTI or bacteremia 22 (36.1) 6 (21.4)
Immunosuppression 3 (4.9) 1 (3.6)
Active malignancy 1 (1.6) 2 (7.1)
Diabetes 23 (37.7) 13 (46.4)
Cirrhosis 3 (4.9) 4 (14.3)
a Temperature >= 38 ?C either measured in the emergency department or prior to arrival.
Any new neurologic deficit 22 (36.1) 8 (28.6)
patients with SEA, a minority had a history of IVDU (13.1%) or any mea- sured fever (32.8%) either in the ED or prior to arrival. Diabetes (46.4%) and recent spinal surgery (35.7%) were the most common historical risk factors among patients with non-SEA pyogenic spinal infection. An anal- ysis comparing patients with spinal infections enrolled during study phase two versus phase one (Appendix Table 1) respectively found that patients enrolled after March 2010 were less likely to have a histor- ical risk factor (75.5% versus 92.5%) or fever in the ED (16.3% versus 35.0%).
- Discussion
In this single center, prospective, community ED cohort of adults in whom the ED provider had clinical concern for pyogenic spinal infec- tion, the clinical characteristics most strongly associated with pyogenic spinal infection were male sex, fever, and recent soft tissue infection or bacteremia. Our study adds to the literature as a large prospective ED cohort evaluating which clinical characteristics are actually associated with spinal infection.
The majority of spinal infections occur due to hematogenous spread, so a recent infection complicated by bacteremia can increase a patient’s risk of spinal infection [4]. The presence of another site of infection was strongly associated with SEA in a large ED-based cohort (OR 18.1) from University of California San Diego (UCSD) and a recent large single cen- ter retrospective study (OR 6.1) [11,32]. Our study reinforces the find- ings of these previous studies that pyogenic spinal infection should be strongly considered when back pain develops after soft tissue infection or any other infection possibly complicated by bacteremia.
Our study finding of male predominance (69.7%) among patients with pyogenic spinal infection is consistent with the largest review of SEA cases (64% male), the UCSD ED cohort (60% male), and a large RCT examining antibiotic regimens for pyogenic vertebral osteomyelitis (69% male) [11,12,21]. To our knowledge, our study is the first to show male sex as a risk factor for pyogenic spinal infection among ED patients. A possible explanation for this finding is that female patients with spinal infections were not identified by ED providers for study inclusion. Alter- natively, males may be at higher risk due to having more risk factors than females. Further study is needed to characterize the presentation of pyogenic spinal infection among females and validate our finding of male sex as a risk factor in other ED settings.
New neurologic deficits were weakly associated with pyogenic spi- nal infection due to the high prevalence of neurologic deficits among enrolled patients with spinal diagnoses other than infection. Radicular pain or midline spinal tenderness were present in many cases of spinal infection, but these findings did not differentiate spinal infection from nonspecific back pain syndromes. Severe pain limiting the ability to transfer from supine to upright sitting position for back examination without assistance was associated with pyogenic spinal infection in our study. Additional study of this finding is needed to determine interrater reliability and validity in other settings. Pyogenic spinal infec- tion is a rare complication of epidural catheters as one prospective study found that SEA complicated one of every 1930 procedures [33]. Addi- tional study is needed for patients presenting to the ED with spinal pain following epidural steroid injection or epidural catheter placement to determine whether these patients are truly at increased risk for pyo- genic spinal infection following these procedures.
All patients with SEA had at least one historical risk factor in the UCSD ED cohort [11]. The minority of SEA patients with no identifiable historical risk factor in our study (19.7%) is consistent with other co- horts [12,34]. The prevalence of IVDU in this UCSD cohort (60%) was much higher than the IVDU prevalence among patients with SEA in our study (13.1%), a review of 915 SEA cases (8.8%), and 162 SEA cases from a Boston academic hospital (20.4%) [11,12,32]. Our data sug- gest that lack of historical risk factors does not definitively exclude SEA and many ED patients presenting with SEA lack IVDU as a risk factor.
Fever has been traditionally reported as present at the time of diag- nosis in the majority of patients with SEA (66% of 915 SEA cases) [12]. In contrast, a minority of patients (7.3%) presented with fever in the UCSD ED cohort, suggesting that fever may be less prevalent among patients with this disease process in the modern ED setting [11]. Indeed, the prevalence of fever in the ED among patients with SEA in our study was only 19.7% and only rose to 32.8% when including patients with fever measured prior to arrival. The lower prevalence of fever in more recent ED-based studies may represent identification of SEA earlier in the course of disease. Regardless, emergency physicians should main- tain SEA on the differential diagnosis in afebrile patients.
Limitations
Initial medical evaluations frequently miss spinal infections [4,14], and we were unable to include patients that did not undergo work up for this disease process in our study. Next, we collected our data at a sin- gle center, so the generalizability of our study to other ED settings is not known. We lack data describing patients when ED providers contacted the PI for enrollment during time periods when the PI was unavailable. Also, we collected data over an extended time period. This is common practice in SEA research given the rarity of the diagnosis, but changing diagnostic, treatment, and epidemiologic patterns may have led to evo- lution of cohort characteristics over the course of the study [6,11,27]. Moreover, there was a change in study enrollment procedures during the study time period. Prior to March 2010, we collected data before the establishment of spinal infection diagnosis. Beginning in April 2010, we collected data after spinal infection was diagnosed. Univariate and multivariate analyses presented in the manuscript only included patients from phase 1 to eliminate this source of bias.
Interpretation of the physical exam for patients with back pain re- quires clinical judgement. A specific example is whether midline spinal tenderness is present in the setting of diffuse, severe back pain. A single attending emergency physician made these assessments, so we are un- able to assess interrater reliability across multiple ED providers. We lack data on post-void residuals as an objective measurement for urinary re- tention, which is reported in 9-24% of SEA cases [12,14,32]. Finally, the diagnosis of spinal infection could be made in one of three ways: MRI, operative findings, or needle aspiration culture. Although our study lacked a single gold standard for diagnosis, we feel that these methods provided the most accurate categorization of subjects and reflection of clinical practice.
- Conclusion
In this prospective cohort of adults with neck or back pain present- ing to a community ED, the clinical characteristics most strongly associ- ated with pyogenic spinal infection were male sex, fever, and recent soft tissue infection or bacteremia. Most patients with spinal epidural ab- scess were afebrile on presentation.
Prior presentations
ACEP Research Forum Abstract #533; October 3, 2018; San Diego,
CA.
Disclaimers
The views expressed herein are those of the authors and do not re-
flect the official policy or position of Brooke Army Medical Center, the
U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, the Department of the Air Force and Department of Defense or the U.S. Government. This original contri- bution has not been published, it is not under consideration for publica- tion elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried
out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically with- out the written consent of the copyright-holder.
Author contributions
WTD, SM, and SS were responsible for study concept and design. WTD and SS were responsible for acquisition of data. All authors were responsible for critical revision of the article for important intellectual content. WTD, MDA, and SS were responsible for analysis and interpre- tation of data. WTD was responsible for drafting the manuscript. WTD, MDA, and SS were responsible for statistical analysis. WTD had full ac- cess to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. WTD takes responsibil- ity for the paper as a whole.
Declaration of Competing Interest
WTD, MDA, SM, BL, SS report no conflicts of interest.
Appendix A
Appendix Table 1
Comparison of patients with pyogenic spinal infection enrolled before and after March 2010 change in study protocol.
Enrolled 2004 to Mar Enrolled Apr 2010 to 2010 (n = 40) 2018 (n = 49)
Spinal epidural abscess present 27 (67.5) 34 (69.4)
Median age (IQR), y 51.5 (41.8 to 59.3) 57 (51 to 64)
Male sex 30 (75) 32 (65.3)
Historical risk factors
>=1 risk factor present 37 (92.5) 37 (75.5)
IVDU history 3 (7.5) 5 (10.2)
Indwelling vascular catheter 4 (10.0) 7 (14.3)
|
Recent SSTI or bacteremia |
15 (37.5) |
13 (26.5) |
|
2 (5.0) |
2 (4.1) |
|
|
Active malignancy |
2 (5.0) |
1 (2.0) |
|
Diabetes |
17 (42.5) |
19 (38.8) |
|
Cirrhosis |
3 (7.5) |
4 (8.2) |
Spinal implant present 0 (0) 2 (4.1)
Recent vertebral fracture 0 (0) 0 (0)
Recent spinal surgery 14 (35.0) 6 (12.2)
Recent spinal injection 0 (0) 9 (18.4) Reported symptoms
Radicular pain 17 (42.5) 28 (57.1)
Urinary incontinence 8 (20.0) 7 (14.3)
History of measured fever 19 (47.5) 9 (18.4) Physical exam findings
|
Fever in ED |
14 (35.0) |
8 (16.3) |
|
11 (27.5) |
19 (38.8) |
|
|
Inability to sit independently |
15 (37.5) |
23 (46.9) |
|
Extremity weakness |
9 (22.5) |
8 (16.3) |
|
6 (15.0) |
4 (8.2) |
|
|
Abnormal reflex exam |
5 (12.5) |
4 (8.2) |
|
15 (37.5) |
15 (30.6) |
- Hadjipavlou AG, Mader JT, Necessary JT, et al. Hematogenous pyogenic spinal infec- tions and their surgical management. Spine (Phila Pa 1976) 2000;25(13):1668-79.
- Babic M, Simpfendorfer CS. Infections of the spine. Infect Dis Clin North Am 2017;31 (2):279-97.
- Darouiche RO. Spinal epidural abscess. N Engl J Med 2006;355(19):2012-20.
- Pradilla G, Ardila GP, Hsu W, et al. Epidural abscesses of the CNS. Lancet Neurol 2009;8(3):292-300.
- Shweikeh F, Saeed K, Bukavina L, et al. An institutional series and contemporary re- view of bacterial spinal epidural abscess: current status and future directions. Neurosurg Focus 2014;37(2):E9.
- Edlow JA. Managing nontraumatic Acute back pain. Ann Emerg Med 2015;66(2): 148-53.
- Pope JV, Edlow JA. Avoiding misdiagnosis in patients with neurological emergencies. Emerg Med Int 2012;2012:1-10.
- Singleton J, Edlow JA. Acute nontraumatic back pain. Emerg Med Clin North Am 2016;34(4):743-57.
- Alerhand S, Wood S, Long B, et al. The time-sensitive challenge of diagnosing spinal epidural abscess in the emergency department. Intern Emerg Med 2017;12(8): 1179-83.
- Davis DP, Salazar A, Chan TC, et al. Prospective evaluation of a clinical decision guide- line to diagnose spinal epidural abscess in patients who present to the emergency department with Spine pain. J Neurosurg Spine 2011;14(6):765-70.
- Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev 2000;23(4):175-204 [discussion 205].
- Bond A, Manian FA. Spinal epidural abscess: a review with special emphasis on ear- lier diagnosis. Biomed Res Int 2016;2016:1-6.
- Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med 2004;26(3):285-91.
- Acharya J, Gibbs WN. Imaging spinal infection. Radiol Infect Dis 2016;3(2):84-91.
- El Sayed M, Witting MD. Low yield of ED magnetic resonance imaging for suspected epidural abscess. Am J Emerg Med 2011;29(9):978-82.
- Madhuripan N, Hicks RJ, Feldmann E, et al. A protocol-based approach to spinal epi- dural abscess imaging improves performance and facilitates early diagnosis. J Am Coll Radiol 2018;15(4):648-51.
- Butler JS, Shelly MJ, Timlin M, et al. Nontuberculous pyogenic spinal infection in adults. Spine (Phila Pa 1976) 2006;31(23):2695-700.
- Nather A, David V, Hee H, et al. Pyogenic vertebral osteomyelitis: a review of 14 cases. J Orthop Surg 2005;13(3):240-4.
- Mylona E, Samarkos M, Kakalou E, et al. Pyogenic vertebral osteomyelitis: a system- atic review of clinical characteristics. Semin Arthritis Rheum 2009;39(1):10-7.
- Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015;385(9971):875-82.
- Zimmerli W. Vertebral osteomyelitis. N Engl J Med 2010;362(11):1022-9.
- Elm E von, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335(7624):806-8.
- Takahashi J, Ebara S, Kamimura M, et al. Early-phase enhanced inflammatory reac- tion after spinal instrumentation surgery. Spine (Phila Pa 1976) 2001;26(15): 1698-704.
- Rosahl SK, Gharabaghi A, Zink P-M, et al. Monitoring of Blood parameters following anterior cervical fusion. J Neurosurg Spine 2000;92(2):169-74.
- Meyer B, Schaller K, Rohde V, Hassler W. The C-reactive protein for detection of early infections after lumbar microdiscectomy. Acta Neurochir 1995;136(3-4):145-50.
- Hlavin ML, Kaminski HJ, Ross JS, et al. Spinal epidural abscess: a ten-year perspec- tive. Neurosurgery 1990;27(2):177-84.
- Lu C-H, Chang W-N, Lui C-C, et al. Adult spinal epidural abscess: clinical features and prognostic factors. Clin Neurol Neurosurg 2002;104(4):306-10.
- Tang H-J, Lin H-J, Liu Y-C, et al. Spinal epidural abscess-experience with 46 patients and evaluation of prognostic factors. J Infect 2002;45(2):76-81.
- Nussbaum ES, Rigamonti D, Standiford H, et al. Spinal epidural abscess: a report of 40 cases and review. Surg Neurol 1992;38(3):225-31.
- Gasbarrini AL, Bertoldi E, Mazzetti M, et al. Clinical features, diagnostic and thera- peutic approaches to haematogenous vertebral osteomyelitis. Eur Rev Med Pharmacol Sci 2005;9(1):53-66.
- Artenstein AW, Friderici J, Holers A, et al. Spinal epidural abscess in adults: a 10-year clinical experience at a Tertiary Care Academic Medical Center. Open forum Infect Dis 2016;3(4):ofw191.
- Wang LP, Hauerberg J, Schmidt JF. Incidence of spinal epidural abscess after epidural analgesia: a national 1-year survey. Anesthesiology 1999;91(6):1928-36.
- Patel AR, Alton TB, Bransford RJ, et al. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J 2014;14 (2):326-30.
