The Cardiac Arrest Sonographic Assessment (CASA) exam - A standardized approach to the use of ultrasound in PEA
Correspondence / American Journal of Emergency Medicine 36 (2018) 715-732 729
Centers for Disease Control and Prevention. CDC Fact Sheet: Reported STDs in the United States - 2015 National Data for Chlamydia, Gonorrhea, and Syphilis. Retrieved from https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/STD-Trends-508.pdf; 2016.
mydia and gonorrhea: U.S. preventive services task force recommendation statement. Ann Intern Med 2014;161:902-10.
Goyal M, McCutcheon M, Hayes K, et al. Sexual history documentation in adolescent emergency department patients. Pediatrics 2011;128:86-91.
taking. Clin Pediatr 1999;38:227-33.
 The Cardiac Arrest Sonographic Assessment
The Cardiac Arrest Sonographic Assessment
(CASA) exam - A standardized approach to the use of ultrasound in PEA?
Background
Emergency physicians (EPs) have started integrating point-of-care- ultrasound (POCUS) into the evaluation of patients with cardiac arrest to identify Reversible causes of Pulseless electrical activity and it is now recommended by the AHA [1-6]. However, POCUS prolongs CPR pauses which negatively impacts survival [7-10]. While POCUS pro- tocols for cardiac arrest have been suggested, they are too complex and cumbersome [2-4,11-12]. Here we present an evidence based, three step protocol that is simple, rapid, and allows the EP to quickly and ac- curately identify reversible causes of PEA while minimizing CPR interruptions.
The CASA exam
The Cardiac Arrest Sonographic Assessment (CASA) exam consists of three, b 10 second POCUS exams to occur at Pulse checks (Fig. 1). We recommend the recorder time each pause out loud. A phased array (car- diac) transducer should be used and all images should be recorded for review. The EP should utilize the optimal cardiac window based on the patient, with the subxiphoid view often being preferable as this view can be performed during ongoing CPR. While the CASA exam is de- signed for a single provider, a second provider should perform the ultra- sound when possible, as this has been associated with shorter CPR interruptions [8]. FAST and Tension pneumothorax exams can be per- formed on a case-by-case basis during CPR.
Step one: cardiac tamponade
Pericardial effusion causing cardiac tamponade is the cause of cardi- ac arrest in 4-15% of patients [5,13-16]. Tamponade can be rapidly in- tervened upon to resolve PEA and patients with tamponade have higher survival to hospital discharge rates (15.4%) than other causes of PEA (1.3%) [5]. The examiner should assess for pericardial effusion and, if present, assess for signs of tamponade such as early diastolic Right ventricular collapse (Fig. 2). If cardiac tamponade is present, a pericardiocentesis should be strongly considered.
Step two: pulmonary embolus
Pulmonary embolism (PE) is the cause of cardiac arrest in 4.0-7.6% patients and these patients have higher Survival to hospital discharge rates (6.7%) than other patients in PEA (1.3%) [5,17-20]. Thus, it is im- portant that EPs quickly and accurately assess for PE in all cardiac arrest patients. To assess for the presence of PE, the examiner should evaluate for signs of right heart strain such as a dilated right ventricle and a com- paratively small left ventricle (Fig. 3). If signs of right heart strain are present, PE should remain a consideration as the cause of PEA.
? Funding sources/disclosures: none.

Fig. 1. The Cardiac Arrest Sonographic Assessment (CASA) exam schematic.
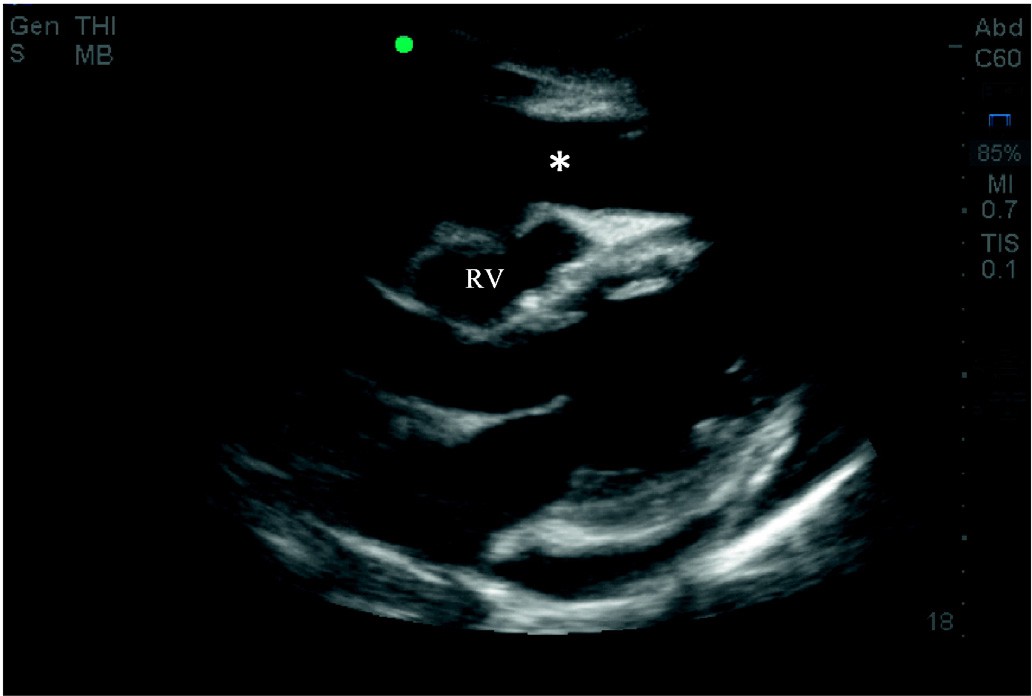
Fig. 2. Parasternal long view demonstrating pericardial effusion (*) causing cardiac tamponade. Note the early diastolic right ventricular collapse (RV).

Fig. 3. Apical four chamber view demonstrating right heart strain consistent with the pres- ence of PE. Note the dilated right atrium (RA) and right ventricle (RV).
730 Correspondence / American Journal of Emergency Medicine 36 (2018) 715-732
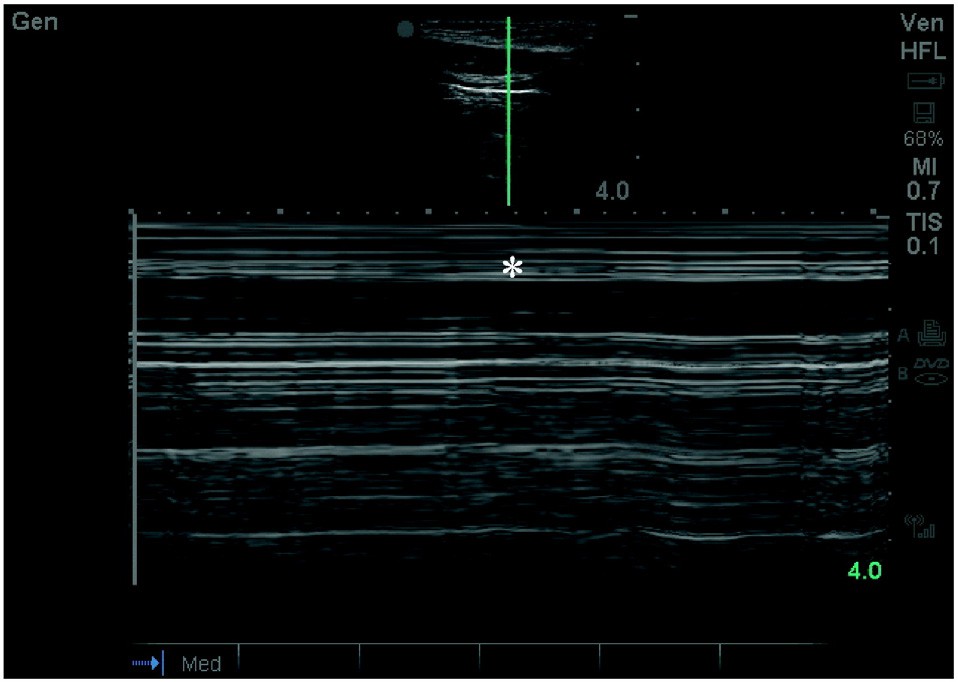
Fig. 4. US image of pneumothorax. Note the characteristic absence of Lung sliding and the bar code sign in M mode (*).
Step three: Cardiac activity
The presence or absence of cardiac activity on bedside echo provides useful prognostic information for patients in PEA [5]. The absence of car- diac activity in PEA portends worse outcomes. Patients in PEA with car- diac standstill on ultrasound have survival to hospital discharge rates ranging from 0.0% to 0.6% [5,21-22]. While low, these rates are not zero and thus Initial resuscitation should be attempted in all patients re- gardless of cardiac activity. The examiner should assess for global cardi- ac activity or fibrillations (Supplemental video 1). If cardiac activity is present, the examiner should reassess for pulses and blood pressure and consider starting continuous vasopressors. If no cardiac activity is seen, the utility of ongoing resuscitation should be reviewed in combi- nation with other poor prognostic factors, such as low end-tidal CO2, prolonged downtime, and unwitnessed arrest.
Ancillary steps: tension pneumothorax and FAST
Tension pneumothorax is an exceedingly rare cause of non-traumat- ic cardiac arrest and can often be diagnosed clinically [18]. During ongoing CPR, the EP should examine the bilateral anterior chest for the absence of lung sliding indicating pneumothorax (Fig. 4). If a pneu- mothorax is detected, needle decompression or thoracostomy may be considered. Small, clinically insignificant pneumothoraces can occur from rib fractures during CPR and clinicians should be aware that these injuries may not need acute intervention.
Also during ongoing CPR, the EP, if the clinical scenario indicates, can assess for a ruptured Abdominal aortic aneurysm (AAA) or ectopic preg- nancy by performing a FAST exam looking for evidence of free fluid [23]. Evaluation of the IVC and hypovolemia was intentionally excluded from the CASA exam as intravenous fluids are traditionally given empir- ically in cardiac arrest and we expect the IVC to be distended in most
cardiac arrest as there is severely limited forward flow.
Discussion
POCUS is a powerful tool for assessing reversible causes of cardiac ar- rest. However, it must be utilized in a protocolized, efficient manner so as to not do harm. The CASA exam assesses for the highest yield revers- ible causes of PEA that can be visualized with ultrasound, while limiting POCUS’s negative impact. It is our hope that the utilization of the CASA exam will limit prolonged CPR pauses and improve diagnostic accuracy. Supplementary data to this article can be found online at http://dx.
doi.org/10.1016/j.ajem.2017.08.052.
Kevin F. Gardner, MD* Eben J. Clattenburg, MD, MPH
Peter Wroe, MD Amandeep Singh, MD Daniel Mantuani, MD
Department of Emergency Medicine, Highland Hospital—Alameda Health
System, Oakland, CA, United States
*Corresponding author at: Highland Hospital, Department of Emergency Medicine, 1411 East 31st St., Oakland, CA 94602,
United States.
E-mail address:[email protected] (K.F. Gardner).
Arun Nagdev, MD
Department of Emergency Medicine, Highland Hospital—Alameda Health
System, Oakland, CA, United States School of Medicine, University of California, San Francisco, CA, United States
19 August 2017
http://dx.doi.org/10.1016/j.ajem.2017.08.052
References
- Tsou PY, Kurbedin J, Chen YS, Chou EH, Lee MG, Lee MC, et al. Accuracy of point-of- care focused echocardiography in predicting outcome of resuscitation in cardiac ar- rest patients: a systematic review and meta-analysis. Resuscitation May 2017;114: 92-9. https://doi.org/10.1016/j.resuscitation.2017.02.021 [Epub 2017 Mar 2. Review].
- Breitkreutz R, Walcher F, Seeger FH. Focused echocardiographic evaluation in resus-
citation management: concept of an advanced life support-conformed algorithm. Crit Care Med May 2007;35(5 Suppl):S150-61 [Review].
Hernandez C, Shuler K, Hannan H, Sonyika C, Likourezos A, Marshall J. C.A.U.S.E.: car- diac arrest ultra-sound exam-a better approach to managing patients in primary non-arrhythmogenic cardiac arrest. Resuscitation Feb 2008;76(2):198-206 [Epub 2007 Sep 5. Review].
- Testa A, Cibinel GA, Portale G, et al. The proposal of an integrated ultrasonographic
approach into the ALS algorithm for cardiac arrest: the PEA protocol. Eur Rev Med Pharmacol Sci 2010;14:77-88.
Gaspari R, Weekes A, Adhikari S, Noble VE, Nomura JT, Theodoro D, et al. Emergency
department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Re- suscitation Dec 2016;109:33-9. https://doi.org/10.1016/j.resuscitation.2016.09.018 [Epub 2016 Sep 28].
Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, et al. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association Guide- lines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132(18 Suppl 2):S444-64.
- Cunningham LM, Mattu A, O’Connor RE, Brady WJ. Cardiopulmonary resuscitation
for cardiac arrest: the importance of uninterrupted chest compressions in cardiac ar- rest resuscitation. Am J Emerg Med Oct 2012;30(8):1630-8. https://doi.org/10. 1016/j.ajem.2012.02.015 [Epub 2012 May 23. Review].
Brown D, Clattenburg E, Wroe P, Losonczy L, Singh A, Nagdaev A. Ultrasound use during cardiac arrest is associated with prolonged cardiopulmonary resuscitation pauses: a prospective cohort study. SAEM abstract 2017;40.
- Reed MJ, Gibson L, Dewar A, Short S, Black P, Clegg GR. Introduction of paramedic led echo in life support into the pre-hospital environment: the PUCA study. Resuscita- tion 2017;112:65-9.
- Veld MAHIT, Allison MG, Bostick DS, Fisher KR, Goloubeva OG, Witting MD, et al. Ul- trasound use during cardiopulmonary resuscitation is associated with delays in chest compressions. Resuscitation 2017.
- Niendorff DF, Rassias AJ, Palac R, Beach ML, Costa S, Greenberg M. Rapid cardiac ul- trasound of inpatients suffering PEA arrest performed by nonexpert sonographers. Resuscitation 2005;67:81-7.
- Jensen MB, Sloth E, Larsen KM, Schmidt MB. Transthoracic echocardiography for car- diopulmonary monitoring in intensive care. Eur J Anaesthesiol 2004;21:700-7.
- Tayal VS, Kline JA. Emergency echocardiography to detect pericardial effusion in pa-
tients in PEA and near-PEA states. Resuscitation 2003 Dec;59(3):315-8.
Chardoli M, Heidari F, Rabiee H, Sharif-Alhoseini M, Shokoohi H, Rahimi-Movaghar
V. Echocardiography integrated ACLS protocol versus conventional cardiopulmonary resuscitation in patients with pulseless electrical activity cardiac arrest. Chin J Traumatol 2012;15(5):284-7.
Hayhurst C, Lebus C, Atkinson PR, Kendall R, Madan R, Talbot J, et al. An evaluation of echo in life support (ELS): is it feasible? What does it add? Emerg Med J Feb 2011; 28(2):119-21. https://doi.org/10.1136/emj.2009.084202 [Epub 2010 Oct].
- Zengin S, Yavuz E, Al B, Cindoruk S, Altunbas G, Gumusboga H, et al. Benefits of car- diac sonography performed by a non-expert sonographer in patients with non-trau- matic cardiopulmonary arrest. Resuscitation May 2016;102:105-9. https://doi.org/ 10.1016/j.resuscitation.2016.02.025 [Epub 2016 Mar 5].
Correspondence / American Journal of Emergency Medicine 36 (2018) 715-732 731
Breitkreutz R, Price S, Steiger HV, Seeger FH, Ilper H, Ackermann H, et al. Focused echocardiographic evaluation in life support and peri-resuscitation of emergency patients: a prospective trial. Resuscitation Nov 2010;81(11):1527-33. https://doi. org/10.1016/j.resuscitation.2010.07.013.
- Beun L, Yersin B, Osterwalder J, Carron PN. Pulseless electrical activity cardiac arrest: time to amend the mnemonic “4H&4T”? Swiss Med Wkly 2015 Jul 31;145:w14178. https://doi.org/10.4414/smw.2015.14178 [eCollection 2015].
- Kurkciyan I, Meron G, Sterz F, Janata K, Domanovits H, Holzer M, et al. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med May 22 2000;160(10):1529-35.
- Comess KA, DeRook FA, Russell ML, Tognazzi-Evans TA, Beach KW. The incidence of pulmonary embolism in unexplained sudden cardiac arrest with pulseless electrical activity. Am J Med Oct 1 2000;109(5):351-6.
- Salen P, Melniker L, Chooljian C, Rose JS, Alteveer J, Reed J, et al. Does the presence or absence of sonographically identified cardiac activity predict resuscitation outcomes of cardiac arrest patients? Am J Emerg Med Jul 2005;23(4):459-62.
- Blaivas M, Fox JC. Outcome in cardiac arrest patients found to have cardiac standstill on the bedside emergency department echocardiogram. Acad Emerg Med Jun 2001; 8(6):616-21.
- Moore C, Todd WM, O’Brien E, Lin H. Free fluid in Morison’s pouch on bedside ultra-
sound predicts need for operative intervention in suspected Ectopic pregnancy. Acad Emerg Med 2007;14:755-8.
 Soft tissue oxygen saturation to predict admission
Soft tissue oxygen saturation to predict admissionWe thank the authors for their interest in our paper, “Soft tissue ox- ygen saturation to predict admission from the emergency department: A prospective observational study” [1]. We agree that it is important to consider whether soft tissue oxygen saturation offers prognos- tic utility in addition to that provided by pulse oximetry (SpO2) as a rou- tine component of triage vital signs. The authors note that we did not include pulse oximetry in our original logistic regression analysis. In adding pulse oximetry to the model, we chose a cutoff value of <= 95% similar to Mower et al. to reflect a level of hypoxia likely to affect clinical decision making [2]. The odds ratio of Sto2 b 76% for predicting admis- sion in the original analysis was 1.54 (95% confidence interval (CI)
1.19 to 1.98). Upon adding SpO2 <= 95% as a predictor variable to the lo- gistic regression model, the odds ratio remained significant for Sto2 b 76% (OR 1.51, 95% CI 1.17 to 1.93). The odds ratio for SpO2 <= 95% is
1.38 (95% CI 1.03 to 1.84). Most patients with SpO2 <= 95% were assigned to more urgent emergency severity index levels (see Table 1) which likely explains these findings [3].
The authors highlight the value of describing the patients within our
study population who had a low Sto2 but were not identified by pulse oximetry. Three hundred twenty-three patients in our study had Sto2 b 76% and SpO2 N 95%. Given the volume of data required to address this request, we will look to provide a future brief report describing the characteristics of these patients. Our intent is that this will lay the foundation for future studies of the impact of routine Sto2 triage mea- surements on ED patient management and Disposition decisions. Much in the way Mower et al. conducted impact studies of pulse oxim- etry use in the 1990s which have now culminated in the routine mea- surement of pulse oximetry in most ED settings [2,4,5], we encourage
future prospective investigations seeking to establish whether Sto2 data lead to significant changes in ED provider Treatment decisions. Un- fortunately, because we blinded providers in our study to the Sto2 re- sults, our current dataset cannot provide insight into the impact of Sto2 on clinical decision making.
A final note: the positive predictive value for Sto2 b 76% predicting admission in our population was 41.1% as calculated by the authors. The positive predictive value of 50% in the manuscript was incorrect, though the 95% confidence interval of 36% to 46% was accurate. We apologize for this error.
William T. Davis, MD Department of Emergency Medicine, San Antonio Uniformed Services Health Education Consortium, San Antonio, TX, United States Corresponding author at: San Antonio Military Medical Center, Department of Emergency Medicine, 3841 Roger Brooke Dr, Fort Sam
Houston, TX 78234, United States.
E-mail address: [email protected].
Josh Lospinso, DPhil
Portia Statistical Consulting LLC, San Antonio, TX, United States
Robert M. Barnwell, MD John Hughes, MD
Department of Emergency Medicine, San Antonio Uniformed Services Health Education Consortium, San Antonio, TX, United States
Steven G. Schauer, DO
United States Army Institute of Surgical Research, San Antonio, TX, United
States
Thomas B. Smith, MD Michael D. April, MD, DPhil, MSc
Department of Emergency Medicine, San Antonio Uniformed Services Health Education Consortium, San Antonio, TX, United States
26 July 2017
http://dx.doi.org/10.1016/j.ajem.2017.08.059
References
Davis WT, Lospinso J, Barnwell RM, Hughes J, Schauer SG, Smith TB, et al. Soft tissue oxygen saturation to predict admission from the emergency department: a prospec- tive observational study. Am J Emerg Med 2017;35(8):1111-7.
- Mower WR, Sachs C, Nicklin EL, Safa P, Baraff LJ. Effect of routine emergency department triage pulse oximetry screening on medical management. Chest 1995;108(5):1297-302.
- Tanabe P, Gimbel R, Yarnold PR, Kyriacou DN, Adams JG. Reliability and validity of scores on the emergency severity index version 3. Acad Emerg Med 2004;11(1):59-65.
- Mower WR, Myers G, Nicklin EL, Kearin KT, Baraff LJ, Sachs C. Pulse oximetry as a fifth vital sign in emergency geriatric assessment. Acad Emerg Med 1998;5(9):858-65.
 Mower WR, Sachs C, Nicklin EL, Baraff LJ. Pulse oximetry as a fifth pediatric vital sign. Pediatrics 1997;99(5):681-6.
Mower WR, Sachs C, Nicklin EL, Baraff LJ. Pulse oximetry as a fifth pediatric vital sign. Pediatrics 1997;99(5):681-6.
Table 1
Number of admissions stratified by Emergency Severity Index level, soft tissue oxy- gen saturation (Sto2) and pulse oximetry (SpO2).
ESI level Sto2 b 76% Sto2 >= 76%
Emergency department electrocardiogram images sent through the mobile phone: Feasibility and accuracy
In patients with cardiac symptoms, the most commonly performed Diagnostic investigation, after a history and physical examination, is 12-lead Electrocardiography . Depending on the practice setting, emergency department (ED) providers may need to confer with a cardi- ologist who is off-site. It is difficult to establish an exact diagnosis through only a verbal report of the ECG and transmittal of images by fax is time consuming. The fax process can also significantly affect the
SpO2 <= 95%
SpO2 N 95%
SpO2 <= 95%
SpO2 N 95%
N admitted/N total
N admitted/N total
N admitted/N total
N admitted/N total
1
0/0
1/1 (100%)
2/2 (100%)
1/3 (33%)
2
16/21 (76%)
45/69 (65%)
36/47 (77%)
164/333 (49%)
3
19/33 (58%)
71/214 (33%)
40/131 (31%)
325/1408 (23%)
4
0/3 (0%)
2/34 (6%)
0/18 (0%)
15/237 (6%)
5
3/4 (75%)
1/5 (20%)
0/2 (0%)
2/23 (9%)
All patients
38/61 (62%)
120/323 (37%)
78/200 (39%)
507/2004 (25%)

 Soft tissue oxygen saturation to predict admission
Soft tissue oxygen saturation to predict admission