Criterion for initiating hemodialysis based on serum caffeine concentration in treating severe caffeine poisoning

 American Journal of Emergency Medicine 46 (2021) 70-73
American Journal of Emergency Medicine 46 (2021) 70-73
Contents lists available at ScienceDirect
American Journal of Emergency Medicine
journal homepage:
 Criterion for initiating hemodialysis based on serum caffeine concentration in treating severe Caffeine poisoning
Criterion for initiating hemodialysis based on serum caffeine concentration in treating severe Caffeine poisoning
Tomohiro Yoshizawa a,?, Yoshito Kamijo, M.D., Ph.D. a, Tomoki Hanazawa, M.D. a, Kiyotaka Usui, Ph.D. b
a Emergency Center and Poison Center, Saitama Medical university hospital, Moroyama, Iruma-gun, Saitama, Japan
b Department of Forensic Medicine, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan
- Introduction
Caffeine (1,3,7-trimethylxanthine) is a natural alkaloid derived from tea leaves, coffee beans, cacao beans, and kola nuts. Pharmacologically, it has a central nervous system (CNS) stimulating effect, a myocardial stimulating effect, a skeletal muscle stimulating effect, a diuretic effect, and a bronchodilating effect. It is also included as an ingredient of sup- plements and energy drinks to prevent or eliminate drowsiness or fa- tigue, particularly via its CNS stimulating effect [1].
In Japan, caffeinated supplements and energy drinks are easily ob- tainable from stores, vending machines, and online purchase without any restriction. Several severe and fatal cases of ventricular arrhythmia or cardiac arrest, as well as nausea/vomiting, excitement/agitation, or sinus tachycardia, have recently been reported among people who con- sumed large or massive amounts of caffeinated supplements or energy drinks [2-5]. Extracorporeal purification including hemodialysis (HD) have been successful in improving toxic signs and symptoms in such cases [6,7]. However, the criteria for initiating extracorporeal purifica- tion is unclear. Accordingly, we conducted a multicenter prospective study to determine a criterion for initiating HD based on serum caffeine concentration for the treatment of severe caffeine poisoning.
Participants were patients transported by ambulance between Octo- ber 2017 and December 2018 to 10 emergency departments of hospitals that participated in this study after consuming large or massive amounts of supplements or energy drinks containing caffeine as a main ingredient (caffeine dose >=1.0 g). Other pharmacologically less ac- tive ingredients of these supplements and drinks included vitamins, sugars, and amino acids. Patients who reported having ingested these caffeinated products, who were witnessed to have used such products, or who were found possessing residual products at the scene were in- cluded in the study. Patients who ingested large or massive amounts of prescription drug mixtures containing pharmacologically active in- gredients such as acetaminophen or ibuprofen in addition to caffeine,
* Corresponding author at: Emergency Center and Poison Center, Saitama Medical University Hospital, 38 Morohongo, Moroyama, Iruma-gun, Saitama 350-0495, Japan.
E-mail address: [email protected] (T. Yoshizawa).
as well as patients who simultaneously overdosed on other drugs such as benzodiazepines, were excluded. During hospitalization, written in- formed consent was obtained from each patient or their family mem- bers prior to enrollment in this study. After patients were transported to the hospitals, anonymized residual serum samples obtained on hos- pital arrival and completed questionnaires from the emergency depart- ments providing patient information were sent to the Emergency Center & Poison Center, Saitama Medical University Hospital, where serum caffeine concentrations were determined and the data analyti- cally analyzed. The questionnaire included close-ended questions requesting information on the following: patient age and gender, com- mercial names and consumed amounts of caffeinated products, caffeine dose, vital signs and laboratory data on emergency department arrival, clinical signs and symptoms, physical complications during the clinical course, treatments including ventilator, HD, direct hemoperfusion (DHP), and veno-arterial extracorporeal membranous oxygenation (VA-ECMO), and outcomes. The pilot questionnaire was tested to deter- mine the time required for completion and whether it was easily under- stood. Physicians, pharmacists, and researchers of the participating hospitals were asked to identify all patients satisfying the above criteria and to complete the questionnaire for every case they identified. This study was approved by the ethics committees of all participating hospi- tals, including that of Saitama Medical University Hospital (notification number: 16-096-3).
Tachypnea was defined as respiratory rate (RR) >=20 breaths/min; tachycardia as heart rate (HR) >=100 beats/min; depressed conscious- ness as a Glasgow Coma Scale (GCS) of 3 to 14; and hyperthermia as body temperature (BT) >=37.5 ?C. Hypercreatinekinasemia was defined as serum creatine kinase (CK) >=200 IU/L; hyperglycemia as serum glu- cose >=180 mg/dL; hypokalemia as serum K+ <= 3.4 mmol/L; hypoph- osphatemia as serum inorganic phosphate (IP) <=2.4 mg/dL; and hyperlactatemia as serum lactate >=2.0 mmol/L.
-
- Materials and reagents
Standard caffeine, ethanol, acetonitrile, and phosphate-buffered sa- line (PBS, pH 7.2-7.4) were purchased from Wako Pure Chemical Indus- tries (Osaka, Japan). Quick, easy, cheap, effective, rugged, and safe (QuEChERS) pre-packed extraction packets (AOAC 2007.01 method) and Captiva ND Lipid cartridges were purchased from Agilent Technol- ogies (Santa Clara, CA, USA).
https://doi.org/10.1016/j.ajem.2021.03.017 0735-6757/(C) 2021 Published by Elsevier Inc.
-
- Toxicological analysis“>Toxicological analysis
A gas chromatograph-mass spectrometer (GC-MS) (GCMS- QP2020(R), Shimadzu Corporation, Kyoto, Japan) was used to analyze all samples. A SH-Rxi-5Sil column (30 m x 0.25 mm i.d., 0.25 um film thickness; Shimadzu Corporation) was used for GC separation. The GC oven temperature was held constant at 100 ?C for 2 min, and then ramped at 15 ?C/min to a final temperature of 320 ?C (hold time, 4 min). The temperature of the injection port and interface was set at 230 ?C. Helium with a flow rate of 1.5 mL/min was used as a carrier gas. The injection volume was set to 2 uL. The mass spectrometer was operated in the electron impact ionization mode. The m/z values of monitored ions for caffeine were m/z 194, m/z 109, and m/z 55.
Each serum sample was extracted by the QuEChERS method and serum caffeine concentration was analyzed by the standard addition method [8].
-
- Statistical analysis
We referred to published case reports of severe caffeine poisoning and created our own clinical symptom-based criteria, which were used to assign participants into the severe or non-severe group [2-4,9-11]. Participants who presented with cardiopulmonary arrest, systolic blood pressure <= 90 mmHg, heart rate >= 140/min, GCS <=8, ven- tricular arrhythmia such as ventricular tachycardia, or convulsive seizures were defined as being severely poisoned and in need of hemo- dialysis (severe group). The remaining participants were assigned to the non-severe group. The U test or Fisher’s exact test was used to compare mean serum concentrations and other clinical parameters between the two groups. The Shapiro-Wilk test for normality of data revealed that several items had poor normality. Accordingly, the U test was used as a non-parametric test. Fisher’s exact test was used to compare Gender distribution between groups. The significant concentration was set to ? = 0.05 (two-sided), and p < 0.05 was considered statistically signifi- cant. Statistical analyses were performed using SPSS Statistics 23 (IBM Corporation, Armonk, NY, USA).
Binomial logistic regression analysis was performed to analyze
serum concentration distributions between the two groups. The objec- tive variable was 0 for non-severe cases and 1 for severe cases, and serum concentration was used as the explanatory variable. SPSS Statis- tics 23, Minitab16 (Minitab LLC, State College, PA, USA) was used for this analysis.
A total of 30 patients from 10 emergency departments of hospitals in Japan were included in this study as participants. Most participants were young (median age, 23 years; range, 14 to 32 years), and the same number of men and women experienced an acute reaction. All participants (100%) reported having consumed caffeinated tablets, and 4 (13.3%) simultaneously consumed energy drinks or coffee. Caffeine doses could be estimated in 29 participants (96.7%) using information obtainable from commercial names and consumed amounts of caffein- ated products (median, 10.0 g; range, 1.4 g to 83.0 g). While the caffeine dose in the remaining participant was unknown, it was estimated that at least 1.0 g of caffeine was consumed based on his confession that he had taken 10 or more 100 mg caffeinated tablets. We were able to esti- mate the time interval between caffeine consumption and arrival at the hospital for 27 participants (90.0%) (median, 3.0 h; range, 0.8 to 20.0 h).
Two participants exhibited cardiopulmonary arrest on admission, whose vital signs were excluded. Abnormal vital signs and laboratory
data considered common on admission were observed in >=33.3% of par- ticipants, including tachypnea (19/27, 70.4%), tachycardia (16/27, 59.3%), depressed consciousness (12/26, 46.2%), hypercreatinine- kinasemia (10/29, 34.4%), hyperglycemia (17/29, 58.6%), hypokalemia (24/29, 82.8%), hypophosphatemia (7/19, 36.8%), and hyperlactatemia (13/23, 56.5%).
-
- Treatments and outcomes
Nine participants (30.0%) were treated with a ventilator, 13 (43.3%) with extracorporeal purification including HD (8; 26.7%) and DHP (5; 16.7%), and 2 (6.7%) with VA-ECMO. Many participants were also treated with medication, as follows: potassium products including po- tassium chloride and potassium aspartate (8; 26.7%), propofol (5; 17.2%), proton pump inhibitor (omeprazole) (5; 16.7%), benzodiaz- epines including midazolam (4; 13.3%), metoclopramide (4; 13.3%), Beta blockers including landiolol and propranolol (3; 10.0%), magne- sium sulfate (3; 10.0%), fentanyl (2; 6.7%), and others.
Twenty-nine participants (96.7%) were admitted to the hospital, with a median length of stay of 4 days (range, 1 to 42 days), and 16 (53.3%) were admitted to the ICU, with a median length of stay of 72 h (range, 2 to 216 h). Twenty-seven participants (90.0%) recovered
completely, 1 (3.4%) was discharged with Epigastric pain, and 2 (6.7%) who exhibited cardiopulmonary arrest on admission died.
-
- Comparative study of serum caffeine concentration
Table 1 shows a comparison between the severe and non-severe groups according to our criteria. Among the 30 participants, 16 were assigned to the non-severe group and 14 to the severe group according to the defined criteria. Demographics, serum caffeine con- centrations, vital signs, and laboratory data were compared between the two groups, and significant differences were observed in gender (p = 0.026), serum caffeine concentrations (p < 0.001), systolic blood pressure (p = 0.039), pulse rate (p < 0.001), and glucose (p < 0.004).
In order to determine the serum concentration which most clearly separates the severe group from the non-severe group, binomial logistic regression analysis with the severe and non-severe group as the target variable and serum caffeine concentration as the explanatory variable was performed. This analysis yielded a caffeine concentration of
147.43 mg/L for a probability of 0.5 (Fig. 1).
Table 1
Demographics, vital signs, laboratory tests, and toxicological analysis (n = 30).
|
Demographics |
Non-severe (n = 16) |
Severe (n = 14) |
p |
|
Age, years |
21.8 +- 5.0 |
23.14 +- 4.4 |
0.667 |
|
Male |
12 (75.0%) |
4 (28.6%) |
0.026 |
|
Vital signs Respiratly rate |
24.8 +- 14.3 |
24.6 +- 6.2 |
0.422 |
|
Systolic blood pressure (mmHg) |
130.4 +- 13.4 |
108.9 +- 25.8 |
0.039 |
|
Diastolic blood pressure (mmHg) |
66.6 +- 15.7 |
64.2 +- 23.1 |
0.610 |
|
Pulse |
98.0 +- 21.9 |
138.3 +- 32.2 |
<0.001 |
|
GCS (total) |
14.2 +- 1.5 |
12.1 +- 3.9 |
0.077 |
|
Body temperature (?C) |
36.3 +- 0.9 |
36.0 +- 1.2 |
0.422 |
|
Laboratory tests CK (IU/L) |
399.9 +- 710.8 |
982.4 +- 2256.9 |
0.619 |
|
Glu (mg/dL) |
183.6 +- 40.5 |
300.9 +- 111.2 |
0.004 |
|
K (mEq/L) |
2.9 +- 0.6 |
3.0 +- 2.1 |
0.268 |
|
IP (mEq/L) |
2.2 +- 1.0 |
4.8 +- 3.5 |
0.156 |
|
Lactic acid (mmol/L) |
2.6 +- 2.85 |
5.9 +- 6.4 |
0.190 |
|
Toxicological analysis Serum caffeine concentration (mg/L) |
83.4 +- 53.3 |
240.6 +- 129.6 |
<0.001 |
GCS:Glasgow Coma Scale, CK: Creatinine kinase, Glu: Serum glucose, IP: inorgic phosphate.
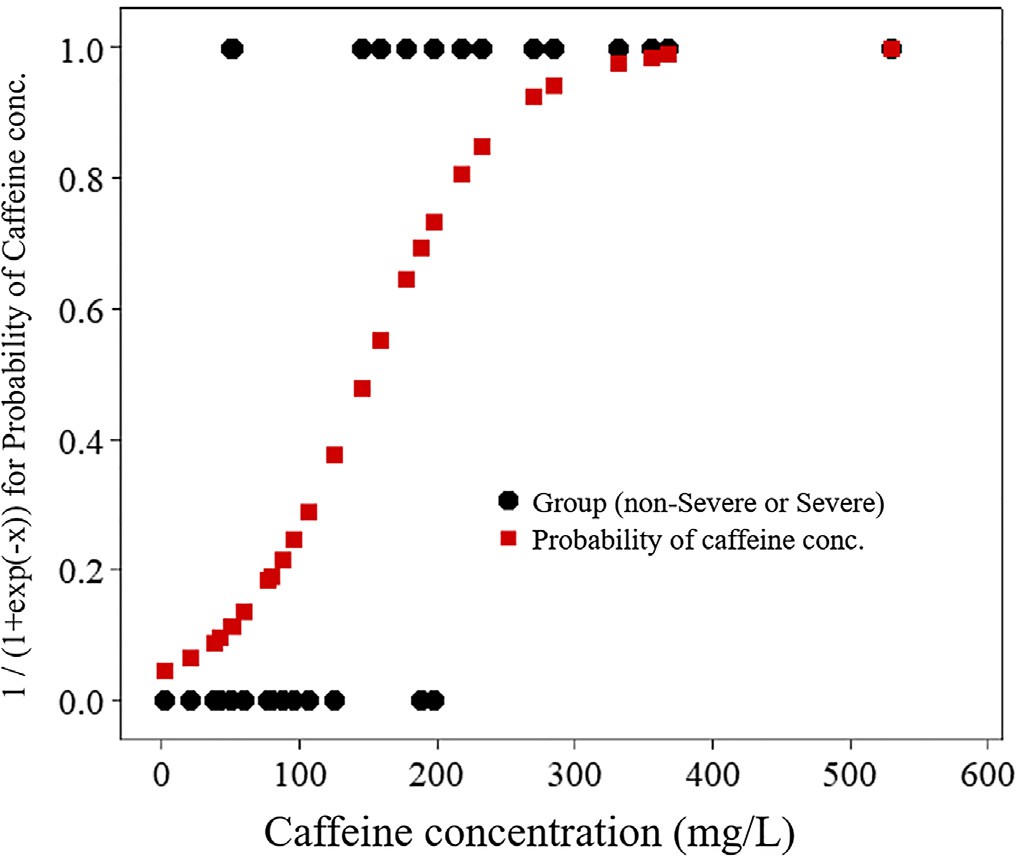
Fig. 1. Binomial logistic regression analysis with severe and non-severe groups as the target variable and caffeine concentration as the explanatory variable.
- Discussion
Theophylline (1,3-dimethylxanthine), a natural alkaloid chemically and pharmaceutically similar to caffeine (1,3,7-trimethylxanthine), is usually prescribed as a sustained release or enteric coated formulation and has a low molecular weight (180 Da), a small distribution volume of approximately 0.5 L/kg, a low protein binding rate of 40 to 60%, a long elimination half-life of 10 to 15 h, and an ability to be absorbed by activated charcoal at a high rate. Theophylline has been used to treat pulmonary diseases such as bronchial asthma and chronic obstruc- tive pulmonary disease (COPD). DHP may enhance the excretion of the- ophylline from the body, with a reported mean clearance with DHP exceeding 100 mL/min [12]. In addition, given its low protein binding rate, theophylline may be dialyzable [13]. The EXTRIP Working Group reported criteria for initiating extracorporeal purification for acute ex- posure to theophylline >100 mg/L (555 mmol/L) [12].
On the other hand, no criterion has been set for initiating extracorpo- real purification based on serum caffeine concentration [6]. After inges- tion, caffeine is rapidly absorbed from the stomach and intestines and the peak concentrations in blood and plasma are reached by 30-90 min [14]. Almost 100% of the caffeine is absorbed into the body, metabolized by CYP1A2 in the liver, and mostly excreted in urine [15]. Approximately 0.5% to 2% of the unchanged drug is excreted in urine [16]. Caffeine has a low molecular weight of 194 Da, a small distribution volume of 0.5 to 0.7 L/kg, a low protein binding rate of approximately 35%, a relatively long elimination half-life of 3 to 10 h, and an ability to be absorbed by activated charcoal at a high rate [12,17]. Similar to the- ophylline, DHP may be effective for enhancing the excretion of caffeine from the body, and at least one study has shown that DHP can shorten the elimination half-life of caffeine [18]. Since caffeine has a lower pro- tein binding rate than theophylline, it also may be more dialyzable [19]. We previously reported two cases of severe caffeine poisoning, with one patient treated by HD and the other by DHP [5]. Serial caffeine con- centrations were measured in those patients to estimate caffeine clear- ance and determine which of HD and DHP is superior for treating severe caffeine poisoning. In both patients, toxic symptoms such as vomiting, tachycardia, and agitation subsided promptly together with a reduction in serum caffeine concentrations. In that study, we con- cluded that HD may be superior to DHP for treating severe caffeine poi- soning, given the higher rate of associated complications including thrombocytopenia, higher costs, fewer skilled experts, and lower clear-
ance rate associated with DHP.
In the present study, we performed one-dimensional binomial logistic regression analysis and found that the concentration of serum caffeine that most clearly separated the severe group from the non- severe group was 147.43 mg/L when using our criterion. Therefore, we recommend initiating HD to treat severe caffeine poisoning when pa- tients exhibit a serum caffeine concentration >= 140 mg/L. This concen- tration does not always reflect the peak concentration, however. We found that it took a median of 3 h to transport patients to the hospital, and by the time the patients arrived, the peak concentration had passed in most cases. The concentration of >=140 mg/L is somewhat higher than currently reported lethal serum caffeine concentrations (80-100 mg/L) and recommended serum theophylline concentrations (>100 mg/L) for initiating extracorporeal purification by the EXTRIP Working Group. We previously reported that the number of patients transported to emergency facilities after large or massive consumption of caffeinated supplements or energy drinks markedly increased since 2013 in Japan, most patients (96%) ingested highly-caffeinated tablets, 6.9% developed cardiac arrest, and 3.0% died with a minimal lethal dose of 6.0 g [2]. All patients in the present study overdosed on caffeinated tablets, with a mortality rate of 6.7%. Most energy drinks commercialized in Japan con- tain <=50 mg/dL of caffeine. Highly-caffeinated tablets may be much more dangerous than energy drinks, as consumption of the minimal lethal dose of caffeine (6.0 g) by ingesting 60 tablets (each containing100 mg) may be much easier than by drinking 12 L of energy drinks (each containing 50 mg/dL of caffeine). Thus, efforts should be made to high- light the risk of ingesting highly-caffeinated tablets. In 2004, Sweden re- stricted the maximum quantity of tablets containing 100 mg caffeine that can be bought over the counter in a single purchase from 250 to 30 tablets, and this measure appeared to be effective in preventing sui- cides [20]. We believe similar restrictions would be appropriate in Japan.
- Limitations
This study has some limitations. First, the sample size was small (n = 30). A larger sample size may be necessary to increase statistical power. Second, we adopted criteria for dividing patients into non- severe and severe groups by referring to previously published case re- ports. The validity of this method and the cut-off we derived from it will need to be confirmed in further studies.
- Conclusion
We recommend initiating HD to treat severe caffeine poisoning when patients present with a serum caffeine concentration >= 140 mg/L. Further larger-scale studies will be needed to confirm our results.
CRediT author statement
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the con- cept, design, analysis, writing, or revision of the manuscript. Further- more, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in the American Journal of Emergency Medicine.
Declaration of Competing Interest
The authors have no interests to declare.
Acknowledgments
The authors thank the emergency departments of participating hos- pitals for providing samples. The authors also thank Dr. S. Ohtsuki for conducting the statistical analysis.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi. org/10.1016/j.ajem.2021.03.017.
References
- Cappelletti S, Piacentino D, Sani G, et al. Caffeine: cognitive and physical perfor- mance enhancer or psychoactive drug? Curr Neuropharmacol. 2015 Jan;13(1): 71-88.
- Kamijo Y, Fujita Y, Usui K. A retrospective study on the epidemiological and clinical features of emergency patients with large or massive consumption of caffeinated supplements or energy drinks in Japan. Intern Med. 2018;57(15):2141-6.
- Ishigaki S, Fukasawa H, Kinoshita-Katahashi N, et al. Caffeine intoxication success- fully treated by hemoperfusion and hemodialysis. Intern Med. 2014;53:2745-7.
- Ishikawa T, Yuasa I, Endoh M. Non specific drug distribution in an autopsy case re- port of fatal caffeine intoxication. Legal Med. 2015;17:535-8.
- Jones AW. Review of caffeine-related fatalities along with postmortem blood con- centrations in 51 poisoning deaths. J Anal Toxicol. 2017;41:167-72.
- Yoshizawa T, Kamijo Y, Hanazawa T, et al. Which of hemodialysis and direct hemoperfusion is more recommended for treating severe caffeine poisoning? Am J Emerg Med. 2019;37(9):1801-2.
- Colin-Benoit E, Friolet R, Rusca M, Teta D, Gobin N. Combination of hemodialysis and hemofiltration in severe caffeine intoxication. Nephrol Ther. 2017 May;13(3):183-7.
- Usui K, Hayashizaki Y, Hashiyada M, et al. Rapid drug extraction from human whole blood using a modified QuEChERS extraction method. Legal Med. 2012;14:286-96.
- Magdalan J, Zawadzki M, Skowronek R, et al. Nonfatal and fatal intoxications with pure caffeine – report of three different cases. Forensic Sci Med Pathol. 2017 Sep; 13(3):355-8.
- Willson C. The clinical toxicology of caffeine: a review and case study. Toxicol Rep. 2018 Nov 3;5:1140-52.
- Cappelletti S, Piacentino D, Fineschi V, Frati P, Cipolloni L, Aromatario M. Caffeine- related deaths: manner of deaths and categories at risk. Nutrients. 2018 May 14; 10(5):611.
- Ghannoum M, Wiegand TJ, Liu KD, Calello DP, EXTRIP workgroup, et al. Extracorpo- real treatment for theophylline poisoning: systematic review and recommendations from the EXTRIP workgroup. Clin Toxicol (Phila). 2015;53(4):215-29.
- Shannon MW. Comparative efficacy of hemodialysis and hemoperfusion in severe Theophylline intoxication. Acad Emerg Med. 1997;4(7):674-8.
- Axelrod J, Reichenthal J. The fate of caffeine in man and a method for its estimation in biological material. J Pharmacol Exp Ther. 1953 Apr;107(4):519-23.
- Bertz RJ, Grannemann GR. Use of in vitro data and in vivo data to estimate the like- lihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32: 210-58.
- Arnaud MJ. Metabolism of caffeine and other components of coffee. In: Garattini S, editor. Caffeine, coffee and health. New York: Raven Press; 1993. p. 43-95.
- Kaur H, Bansiwal A, Hippargi G, et al. Effect of hydrophobicity of pharmaceuticals and personal care products for adsorption on activated carbon: adsorption iso- therms, kinetics and mechanism. Environ Sci Pollut Res Int. 2018 Jul;25(21): 20473-85.
- Regenthal R, Krueger M, Koeppel C, et al. Drug concentration: therapeutic and toxic serum/plasma concentrations of common drugs. J Clin Monit. 1999;15:529-44.
- Kohl BA, Kaur K, Dincher N, et al. Acute intentional Caffeine overdose treated pre- emptively with hemodialysis. Am J Emerg Med. 2020 Mar;38(3) 692.e1-692.e3.
- Thelander G, Jonsson AK, Personne M, et al. Caffeine fatalities- do sales restrictions prevent intentional intoxications? Clin Toxicol. 2010;48:354-8.
